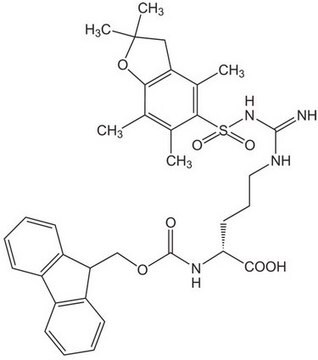
추천 제품
Quality Level
제품 라인
Novabiochem®
분석
≥85.0% (acidimetric)
≥98% (TLC)
≥98.0% (HPLC)
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
mp
70-120 °C (decomposition)
응용 분야
peptide synthesis
작용기
amine
저장 온도
15-25°C
InChI
1S/C35H42N4O7S/c1-20-21(2)31(22(3)23-16-17-35(4,5)46-30(20)23)47(43,44)39-33(36)37-18-10-15-29(32(40)41)38-34(42)45-19-28-26-13-8-6-11-24(26)25-12-7-9-14-27(25)28/h6-9,11-14,28-29H,10,15-19H2,1-5H3,(H,38,42)(H,40,41)(H3,36,37,39)/t29-/m0/s1
InChI key
QTWZCODKTSUZJN-LJAQVGFWSA-N
일반 설명
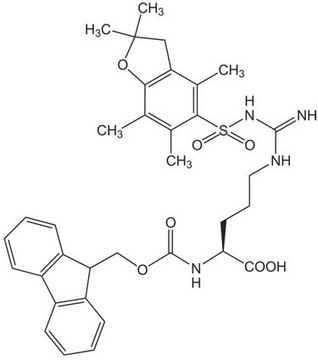
An excellent derivative for the introduction of Arg in Fmoc SPPS. The Pmc group is removed by TFA in 1-3 hours [1,2,3]. For discussions on the side-reactions associated with the use of sulfonyl-based arginine protection, see [4,5,6,7,8,9,10,11]. In the synthesis of peptides containing both Arg and Trp, it is recommended that this derivative is used in conjunction with Fmoc-Trp(Boc)-OH (852050) [5,6,11].
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] R. Ramage, et al. (1987) Tetrahedron Lett., 28, 2287.
[2] J. Green, et al. (1988) Tetrahedron Lett., 29, 4341.
[3] R. Ramage, et al. (1991) Tetrahedron Lett., 47, 6353.
[4] P. Sieber (1987) Tetrahedron Lett., 28, 1637.
[5] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
[6] H. Choi, et al. (1993) Int. J. Peptide Protein Res., 42, 58.
[7] A. Stierandova, et al. (1994) Int. J. Peptide Protein Res., 43, 31.
[8] P. M. Fischer, et al. (1992) Int. J. Peptide Protein Res., 40, 19.
[9] S. A. G. Beck, et al. (1991) Int. J. Peptide Protein Res., 38, 25.
[10] E. Jaeger, et al. (1993) Biol. Chem. Hoppe-Seyler, 374, 349.
[11] P. White in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 537.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] R. Ramage, et al. (1987) Tetrahedron Lett., 28, 2287.
[2] J. Green, et al. (1988) Tetrahedron Lett., 29, 4341.
[3] R. Ramage, et al. (1991) Tetrahedron Lett., 47, 6353.
[4] P. Sieber (1987) Tetrahedron Lett., 28, 1637.
[5] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
[6] H. Choi, et al. (1993) Int. J. Peptide Protein Res., 42, 58.
[7] A. Stierandova, et al. (1994) Int. J. Peptide Protein Res., 43, 31.
[8] P. M. Fischer, et al. (1992) Int. J. Peptide Protein Res., 40, 19.
[9] S. A. G. Beck, et al. (1991) Int. J. Peptide Protein Res., 38, 25.
[10] E. Jaeger, et al. (1993) Biol. Chem. Hoppe-Seyler, 374, 349.
[11] P. White in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 537.
결합
Replaces: 04-12-1073
분석 메모
Color (visual): white to slight yellow to beige
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (TLC(011C)): ≥ 98 %
Purity (TLC(157B)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (12,5 mmol in 25 ml DMF): clearly soluble
Assay (acidimetric): ≥ 85.0 %
Water (K. F.): ≤ 1.50 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (TLC(011C)): ≥ 98 %
Purity (TLC(157B)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (12,5 mmol in 25 ml DMF): clearly soluble
Assay (acidimetric): ≥ 85.0 %
Water (K. F.): ≤ 1.50 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
법적 정보
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.