추천 제품
생물학적 소스
rabbit
Quality Level
항체 형태
affinity purified immunoglobulin
항체 생산 유형
primary antibodies
클론
polyclonal
정제법
affinity chromatography
종 반응성
mouse, rat
제조업체/상표
Chemicon®
기술
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
NCBI 수납 번호
UniProt 수납 번호
배송 상태
dry ice
타겟 번역 후 변형
unmodified
유전자 정보
mouse ... Kcnc1(16502)
특이성
Recognizes a full length Kv3.1b protein. Does not cross react with any other potassium channel antigens tested so far.
면역원
Purified peptide from rat Kv3.1b (amino acids 567-585) (Accession P25122).
애플리케이션
This Anti-Potassium Channel Kv3.1b Antibody is validated for use in IH, IP, WB for the detection of Potassium Channel Kv3.1b.
Western blot: 1:200 using ECL on rat brain membranes.
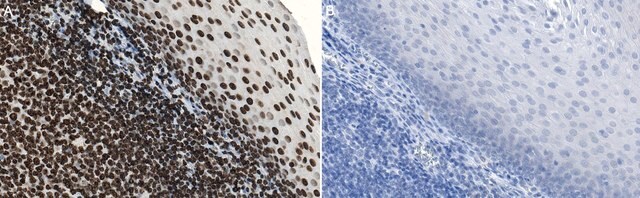
Immunohistochemistry on rat brain sections.
Immunoprecipitation
Dilutions should be made using a carrier protein such as BSA (1-3%)
Optimal working dilutions must be determined by the end user.
PROTOCOL FOR IMMUNOHISTOCHEMISTRY
Sacrifice and tissue processing:
Rats are deeply anesthetized with pentobarbital sodium (Pental). Brain is fixed by transcardial perfusion, first with 50 mL of phosphate buffered saline (0.02M PBS, pH 7.4) containing heparin (5U/ml), then with 220 mL of ice-cold 4% paraformaldehyde in 0.1M PBS, pH 7.5 containing sucrose 4%. Brain is cut in coronal blocks and further fixed by immersion in the same fixative, refrigerated, for 1-2 hours. Brain blocks are transferred to 10% sucrose in 0.1M PBS and sectioned in a cryostat within 3 days. Brain sections, 30 μm thick, are floated in 0.1M PBS and then preserved in a cryopreservation buffer at -20°C. The cryopreservation buffer contains 40% ethylene glycol and 1% polyvinylpyrrolidone in 0.1M potassium acetate buffer, pH 6.5.
Staining procedure:
1. Floating sections are rinsed in 0.02M PBS, 2x 5 minutes.
2. Endogenous peroxidase activity is quenched by incubation with 0.2 % hydrogen peroxide in 0.1M phosphate buffer pH 7.3 containing 0.2% Triton X-100 for 25 minutes at room temperature.
3. Sections are rinsed in 0.02M PBS, 2x 5 minutes.
4. Sections are incubated with the primary antiserum in a medium containing 0.3% Triton X-100, 0.05% Tween 20, 4% normal donkey serum (NDS), for 1 hour at room temperature and then overnight refrigerated.
5. Sections are rinsed in 0.02M PBS, containing 4% NDS, 2x 5 minutes.
Sections are incubated with biotinylated donkey anti-rabbit (from Chemicon USA, catalog number AP182B) diluted 1:400 in 0.02M PBS, containing 0.3% Triton X-100, 0.05% Tween 20, and 4% NDS, for 1 hour at room temperature and then overnight refrigerated.
1. Sections are rinsed in 0.02M PBS containing 4% NDS, 2x 5 min.
2. Sections are incubated with extravidin-peroxidase (Sigma Catalog number E2886) diluted 1:100 in 0.02M PBS, for 45 minutes at room temperature.
3. Sections are rinsed in 0.02M PBS, 3x 5 minutes.
Color development:
1. Sections are incubated with a solution of diaminobenzidine at the concentration of 0.0125% and containing 0.05% nickel ammonium sulfate for 10 minutes at room temperature.
2. Sections are transferred to the same DAB solution but with added hydrogen peroxide at a final concentration of 0.0015%. Duration of incubation should be adjusted by the end user.
3. Sections are rinsed in 0.02M PBS, 4x 10 minutes.
4. Sections are mounted on glass slides (gelatinized or coated by any other type of adhesive material) and allowed to dry.
5. Sections are dehydrated in ascending series of ethanol concentrations (70%, 90%, 100%, 5 minutes in each), delipidated in xylene (10 minutes) and coverslipped in Permount (or any other xylene diluted adhesive).
Immunohistochemistry on rat brain sections.
Immunoprecipitation
Dilutions should be made using a carrier protein such as BSA (1-3%)
Optimal working dilutions must be determined by the end user.
PROTOCOL FOR IMMUNOHISTOCHEMISTRY
Sacrifice and tissue processing:
Rats are deeply anesthetized with pentobarbital sodium (Pental). Brain is fixed by transcardial perfusion, first with 50 mL of phosphate buffered saline (0.02M PBS, pH 7.4) containing heparin (5U/ml), then with 220 mL of ice-cold 4% paraformaldehyde in 0.1M PBS, pH 7.5 containing sucrose 4%. Brain is cut in coronal blocks and further fixed by immersion in the same fixative, refrigerated, for 1-2 hours. Brain blocks are transferred to 10% sucrose in 0.1M PBS and sectioned in a cryostat within 3 days. Brain sections, 30 μm thick, are floated in 0.1M PBS and then preserved in a cryopreservation buffer at -20°C. The cryopreservation buffer contains 40% ethylene glycol and 1% polyvinylpyrrolidone in 0.1M potassium acetate buffer, pH 6.5.
Staining procedure:
1. Floating sections are rinsed in 0.02M PBS, 2x 5 minutes.
2. Endogenous peroxidase activity is quenched by incubation with 0.2 % hydrogen peroxide in 0.1M phosphate buffer pH 7.3 containing 0.2% Triton X-100 for 25 minutes at room temperature.
3. Sections are rinsed in 0.02M PBS, 2x 5 minutes.
4. Sections are incubated with the primary antiserum in a medium containing 0.3% Triton X-100, 0.05% Tween 20, 4% normal donkey serum (NDS), for 1 hour at room temperature and then overnight refrigerated.
5. Sections are rinsed in 0.02M PBS, containing 4% NDS, 2x 5 minutes.
Sections are incubated with biotinylated donkey anti-rabbit (from Chemicon USA, catalog number AP182B) diluted 1:400 in 0.02M PBS, containing 0.3% Triton X-100, 0.05% Tween 20, and 4% NDS, for 1 hour at room temperature and then overnight refrigerated.
1. Sections are rinsed in 0.02M PBS containing 4% NDS, 2x 5 min.
2. Sections are incubated with extravidin-peroxidase (Sigma Catalog number E2886) diluted 1:100 in 0.02M PBS, for 45 minutes at room temperature.
3. Sections are rinsed in 0.02M PBS, 3x 5 minutes.
Color development:
1. Sections are incubated with a solution of diaminobenzidine at the concentration of 0.0125% and containing 0.05% nickel ammonium sulfate for 10 minutes at room temperature.
2. Sections are transferred to the same DAB solution but with added hydrogen peroxide at a final concentration of 0.0015%. Duration of incubation should be adjusted by the end user.
3. Sections are rinsed in 0.02M PBS, 4x 10 minutes.
4. Sections are mounted on glass slides (gelatinized or coated by any other type of adhesive material) and allowed to dry.
5. Sections are dehydrated in ascending series of ethanol concentrations (70%, 90%, 100%, 5 minutes in each), delipidated in xylene (10 minutes) and coverslipped in Permount (or any other xylene diluted adhesive).
분석 메모
Control
CONTROL ANTIGEN: Included free of charge with the antibody is 40 μg of control antigen (lyophilized powder in phosphate buffered saline, pH 7.4, containing 5% sucrose and 0.025% sodium azide). The stock solution of the antigen can be made up using 200 μL of sterile deionized water. For negative control, preincubate 1 μg of peptide with 1 μg of antibody for one hour at room temperature. Optimal concentrations must be determined by the end user.
CONTROL ANTIGEN: Included free of charge with the antibody is 40 μg of control antigen (lyophilized powder in phosphate buffered saline, pH 7.4, containing 5% sucrose and 0.025% sodium azide). The stock solution of the antigen can be made up using 200 μL of sterile deionized water. For negative control, preincubate 1 μg of peptide with 1 μg of antibody for one hour at room temperature. Optimal concentrations must be determined by the end user.
기타 정보
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
법적 정보
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
David Soares et al.
The Journal of comparative neurology, 525(9), 2164-2174 (2017-02-19)
There are substantial differences across species in the organization and function of the motor pathways. These differences extend to basic electrophysiological properties. Thus, in rat motor cortex, pyramidal cells have long duration action potentials, while in the macaque, some pyramidal
Hui Hong et al.
Frontiers in cellular neuroscience, 12, 175-175 (2018-07-13)
In the auditory system, tonotopy is the spatial arrangement of where sounds of different frequencies are processed. Defined by the organization of neurons and their inputs, tonotopy emphasizes distinctions in neuronal structure and function across topographic gradients and is a
Dean O Smith et al.
PloS one, 3(2), e1604-e1604 (2008-02-14)
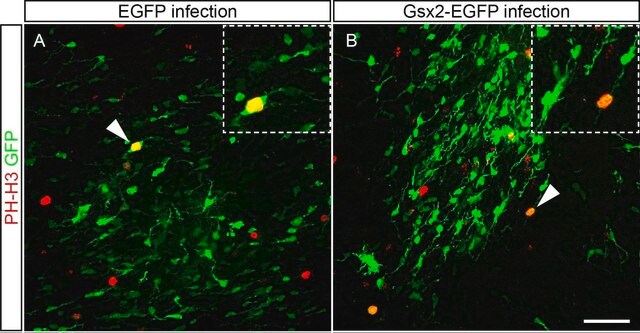
Because of the importance of voltage-activated K(+) channels during embryonic development and in cell proliferation, we present here the first description of these channels in E15 rat embryonic neural progenitor cells derived from the subventricular zone (SVZ). Activation, inactivation, and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.