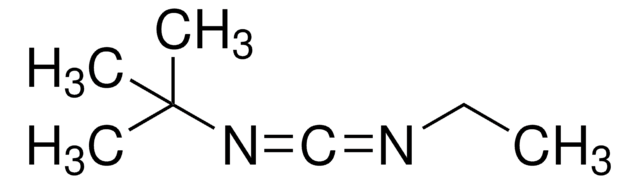모든 사진(1)
About This Item
Linear Formula:
(CH3)2CHN=C=NCH(CH3)2
CAS Number:
Molecular Weight:
126.20
Beilstein:
878281
EC Number:
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.22
추천 제품
product name
DIC, purum, ≥98.0% (GC)
grade
purum
Quality Level
분석
≥98.0% (GC)
형태
liquid
반응 적합성
reaction type: Coupling Reactions
refractive index
n20/D 1.433 (lit.)
bp
145-148 °C (lit.)
density
0.815 g/mL at 20 °C (lit.)
0.815 g/mL at 20 °C
응용 분야
peptide synthesis
작용기
amine
SMILES string
CC(C)N=C=NC(C)C
InChI
1S/C7H14N2/c1-6(2)8-5-9-7(3)4/h6-7H,1-4H3
InChI key
BDNKZNFMNDZQMI-UHFFFAOYSA-N
유전자 정보
human ... EPHX2(2053)
mouse ... Ephx2(13850)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
N,N′-Diisopropylcarbodiimide (DIC) is a carbodiimide used as a coupling reagent in the synthesis of amides, peptides, ureas, heterocycles, and unsymmetrical carbodiimides. It is also used in the polymerization reactions as an activator.
애플리케이션
N,N′-Diisopropylcarbodiimide can be used:
- To synthesize lanthanide (Ln) guanidinate complexes via insertion of carbodiimide into the Ln-N bond of lanthanocene secondary amido complexes.
- To facilitate the cyclization of N-(β-Hydroxy)amides to form 2-oxazolines.
- To synthesize 1-isopropyl-2-alkoxycarbonyl-3-isopropyliminio-aziridine by reacting with alkyl diazoacetates in the presence of transition metal salts.
DIC can be used as:
- A coupling reagent for the synthesis of various esters and amides by treating carboxylic acids with phenols and amines respectively.
- A reagent for the conversion of alcohols to aldehydes or ketones in the presence of DMSO via modified Moffatt-type oxidation reaction.
- A reagent to facilitates the preparation of alkyl halides from corresponding alcohols via the formation of o-alkylisourea.
Alternative to dicyclohexylcarbodiimide in peptide synthesis.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
91.4 °F
Flash Point (°C)
33 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis of iminoaziridines from carbodiimides and diazoesters: a new example of transition metal salt catalysed reactions of carbenes.
Hubert A
Tetrahedron Letters, 17(16), 1317-1318 (1976)
Stefano Crosignani et al.
Chemical communications (Cambridge, England), (2), 260-261 (2003-02-15)
Alcohols can be converted in high yields to the corresponding alkyl halides in a one-pot procedure via the corresponding O-alkylisourea; very short reaction times are possible when microwave irradiation is used.
1, 3-Diisopropylcarbodiimide
Nora GP, et al.
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Amidation and esterification of carboxylic acids with amines and phenols by N, N?-diisopropylcarbodiimide: A new approach for amide and ester bond formation in water
Fattahi N, et al.
Tetrahedron, 74(32), 4351-4356 (2018)
Insertion of a carbodiimide into the Ln? N ?-bond of organolanthanide complexes. Isomerization and rearrangement of organolanthanides containing guanidinate ligands.
Zhang J
Organometallics, 23(13), 3303-3308 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.