추천 제품
Grade
analytical standard
Quality Level
vapor density
2.55 (vs air)
vapor pressure
165 mmHg ( 20 °C)
분석
≥99.9% (GC)
autoignition temp.
936 °F
유통기한
limited shelf life, expiry date on the label
expl. lim.
16 %
기술
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.361 (lit.)
n20/D 1.362
bp
57-58 °C (lit.)
mp
−98 °C (lit.)
density
0.934 g/mL at 25 °C
응용 분야
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
형식
neat
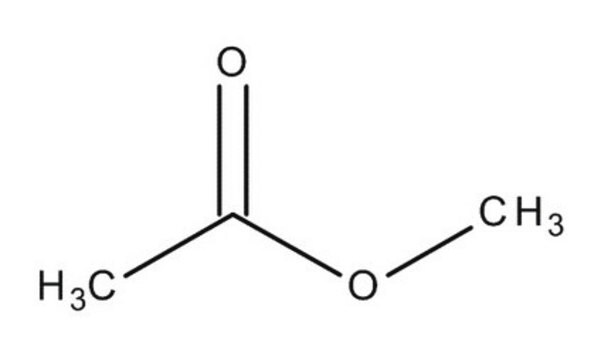
SMILES string
COC(C)=O
InChI
1S/C3H6O2/c1-3(4)5-2/h1-2H3
InChI key
KXKVLQRXCPHEJC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Methyl acetate is an acetate ester, classified as a volatile organic compound (VOC). It is used as a solvent for many resins and oils.
애플리케이션
The analytical standard can also be used as follows:
- Quantitative analysis of seven volatile organic compounds (VOCs) in paint coating samples by dynamic headspace-gas chromatography-mass spectrometry (D-HS-GC-MS)
- Detection of 88 VOCs in breath samples of lung cancer patients using solid-phase microextraction (SPME) combined with GC-MS
- Development of a thermal desorption-gas chromatography-mass spectrometry (TDS-GC/MS) based method for the identification of very volatile organic compounds (VVOCs) in indoor air samples
기타 정보
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
8.6 °F - closed cup
Flash Point (°C)
-13 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
Lei Yang et al.
The journal of physical chemistry. A, 112(28), 6364-6372 (2008-06-21)
The mechanisms and the kinetics of the OH (OD) radicals with methyl acetate CH3C(O)OCH3 are investigated theoretically. The dual-level direct dynamics method is employed in the calculation of the rate constants. The optimized geometries and frequencies and the gradients of
Nicolas Salem et al.
Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging, 13(1), 140-151 (2010-04-20)
Studies have established the value of [(methyl)1-(11)C]-acetate ([(11)C]Act) combined with 2-deoxy-2[(18)F]fluoro-D-glucose (FDG) for detecting hepatocellular carcinoma (HCC) using positron emission tomography (PET). In this study, the metabolic fate of [(11)C]Act in HCC was characterized. Experiments with acetic acid [1-(14)C] sodium
Benjamin Bechem et al.
The Journal of organic chemistry, 75(5), 1795-1798 (2010-02-06)
5-Substituted-2-furan methanols 1a-c are subject to enantioselective carbonyl allylation, crotylation and tert-prenylation upon exposure to allyl acetate, alpha-methyl allyl acetate, or 1,1-dimethylallene in the presence of an ortho-cyclometalated iridium catalyst modified by (R)-Cl,MeO-BIPHEP, (R)-C3-TUNEPHOS, and (R)-C3-SEGPHOS, respectively. In the presence
Saman Mohammadi et al.
PloS one, 9(9), e106653-e106653 (2014-09-11)
In this study, we compared, for the first time, the release of a 432 kDa prostaglandin F2a analogue drug, Latanoprost, from commercially available contact lenses using in vitro models with corneal epithelial cells. Conventional polyHEMA-based and silicone hydrogel soft contact
Marco Candelaresi et al.
The journal of physical chemistry. A, 113(46), 12783-12790 (2009-10-02)
The solvation dynamics of methyl acetate in heavy water are analyzed by means of two-dimensional infrared spectroscopy, in conjunction with Car-Parrinello molecular dynamics simulations. The C horizontal lineO stretching infrared band of methyl acetate in water splits into a doublet
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.