M1824990
Metoclopramide
European Pharmacopoeia (EP) Reference Standard
동의어(들):
4-Amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide, Methoxychloroprocainamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
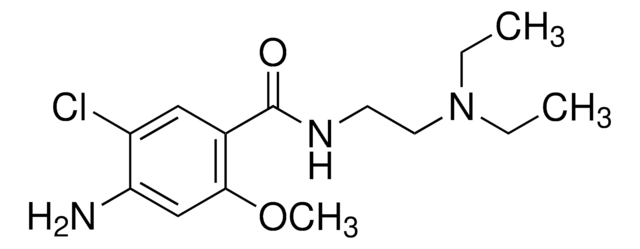
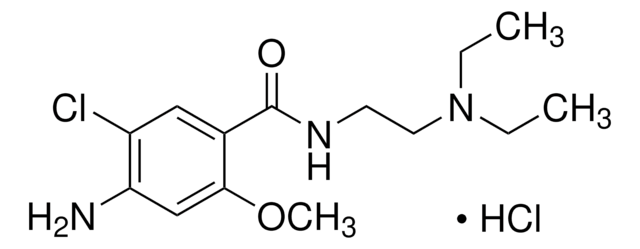
C14H22ClN3O2
CAS Number:
Molecular Weight:
299.80
Beilstein:
1884366
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
metoclopramide
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CCN(CC)CCNC(=O)c1cc(Cl)c(N)cc1OC
InChI
1S/C14H22ClN3O2/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3/h8-9H,4-7,16H2,1-3H3,(H,17,19)
InChI key
TTWJBBZEZQICBI-UHFFFAOYSA-N
유전자 정보
human ... DRD2(1813) , HTR3A(3359) , HTR4(3360)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Metoclopramide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
가장 최신 버전 중 하나를 선택하세요:
Metoclopramide for the treatment of gastroesophageal reflux disease in infants: a systematic review.
Anna Maria Hibbs et al.
Pediatrics, 118(2), 746-752 (2006-08-03)
Metoclopramide is a commonly used drug to treat gastroesophageal reflux disease in infants. Given its widespread use and growing concern about toxicity in this population, we conducted a systematic review of metoclopramide for the treatment of gastroesophageal reflux disease in
P Jay Pasricha et al.
Nature clinical practice. Gastroenterology & hepatology, 3(3), 138-148 (2006-03-03)
Metoclopramide, the only drug approved by the FDA for treatment of diabetic gastroparesis, but used off-label for a variety of other gastrointestinal indications, has many potentially troublesome adverse neurologic effects, particularly movement disorders. In this article, we comprehensively review the
Paul M Simpson et al.
Emergency medicine Australasia : EMA, 23(4), 452-457 (2011-08-10)
The objective of the present study was to conduct a systematic review and meta-analysis of randomized controlled trials, comparing metoclopramide with placebo, for preventing vomiting in patients who have received i.v. morphine for acute pain in the emergency setting, and
William F Malcolm et al.
Clinics in perinatology, 39(1), 99-109 (2012-02-22)
Pharmacotherapy for gastroesophageal reflux (GER) in neonates, aimed at interfering with this physiologic process and potentially reducing the negative sequelae that providers often attribute to GER, consists primarily of drugs that increase the viscosity of feeds, reduce stomach acidity, or
B M Mishriky et al.
British journal of anaesthesia, 108(3), 374-383 (2012-02-07)
Nausea and vomiting occur commonly during and after Caesarean delivery (CD) performed under neuraxial anaesthesia. Metoclopramide is a prokinetic agent reported to be safe in parturients. This meta-analysis assesses the efficacy of metoclopramide for prophylaxis against intra- and postoperative nausea
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.