O0151000
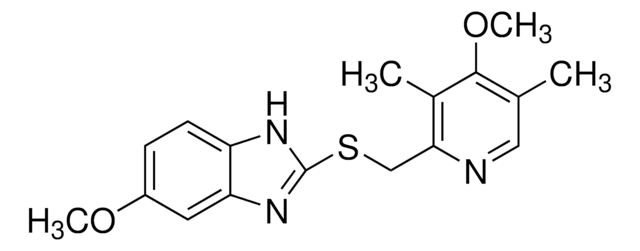
Omeprazole impurity D
European Pharmacopoeia (EP) Reference Standard
동의어(들):
Omeprazole sulfone, 5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfonyl}-1H-benzimidazole, Omeprazole sulphone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C17H19N3O4S
CAS Number:
Molecular Weight:
361.42
Beilstein:
8347309
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
omeprazole
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
O=S(C1=NC2=CC(OC)=CC=C2N1)(CC3=NC=C(C)C(OC)=C3C)=O
InChI
1S/C17H19N3O4S/c1-10-8-18-15(11(2)16(10)24-4)9-25(21,22)17-19-13-6-5-12(23-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)
InChI key
IXEQEYRTSRFZEO-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Omeprazole impurity D EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
A Abelö et al.
Drug metabolism and disposition: the biological fate of chemicals, 28(8), 966-972 (2000-07-20)
This study demonstrates the stereoselective metabolism of the optical isomers of omeprazole in human liver microsomes. The intrinsic clearance (CL(int)) of the formation of the hydroxy metabolite from S-omeprazole was 10-fold lower than that from R-omeprazole. However, the CL(int) value
Ylva Böttiger
European journal of clinical pharmacology, 62(8), 621-625 (2006-06-23)
The hydroxylation of omeprazole, measured as the ratio of omeprazole/5-hydroxyomeprazole in a plasma sample taken 3 h after an oral dose, is an established method to determine CYP2C19 activity, and the ratio of omeprazole AUC/omeprazole sulfone AUC has been used
Ia Hultman et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 848(2), 317-322 (2006-12-05)
A LC-MS/MS method was developed for quantitative determination of esomeprazole, and its two main metabolites 5-hydroxyesomeprazole and omeprazole sulphone in 25 microL human, rat or dog plasma. The analytes and their internal standards were extracted from plasma into methyl tert-butyl
Yanhua Zhang et al.
Journal of clinical pharmacology, 46(3), 345-352 (2006-02-24)
To determine the effects of sex and menstrual cycle phase on CYP3A activity and to characterize the intraindividual variability of CYP3A, 24 Caucasian adults were given a single dose of omeprazole every 14th day for 3 months (men) or during
Naser L Rezk et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 844(2), 314-321 (2006-08-22)
A simple, sensitive and specific reverse-phase high-performance liquid chromatography (HPLC) assay for the simultaneous quantitative determination of omeprazole and its three metabolites in human plasma was developed and validated. This method provides excellent chromatographic resolution and peak shape for the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.