추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1026004
API family
paracetamol, mesalazine, acetaminophen
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
bp
164 °C/11 mmHg (lit.)
mp
120-124 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
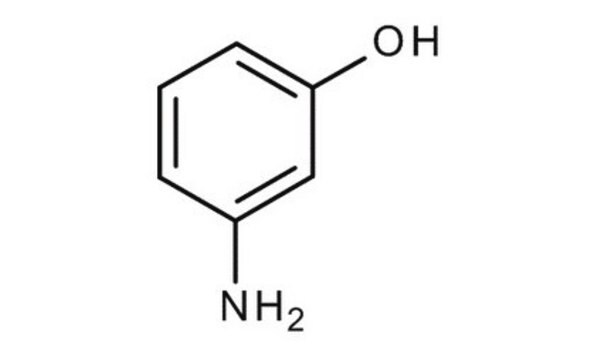
Nc1cccc(O)c1
InChI
1S/C6H7NO/c7-5-2-1-3-6(8)4-5/h1-4,8H,7H2
InChI key
CWLKGDAVCFYWJK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAA9037 in the slot below. This is an example certificate only and may not be the lot that you receive.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Mutsuko Koizumi et al.
The Journal of toxicological sciences, 27(5), 411-421 (2003-01-22)
Repeated dose toxicity of 3-aminophenol was examined on oral administration to newborn and young rats, and susceptibility was analyzed in terms of the no observed adverse effect level (NOAEL) and the unequivocally toxic level. In the 18-day newborn rat study
A new twist to an old tale: novel insights into the differential toxicities of acetaminophen and its regioisomer N-acetyl-meta-aminophenol (AMAP).
J Gerry Kenna
Archives of toxicology, 87(1), 15-18 (2012-09-25)
F Bohnenstengel et al.
Journal of chromatography. B, Biomedical sciences and applications, 692(1), 163-168 (1997-04-25)
A high-performance liquid chromatographic method for the quantification of acrolein following incubation of cyclophosphamide (CP) with human liver microsomes was developed. Based on the formation of the fluorescent derivative 7-hydroxyquinoline by condensation of acrolein with 3-aminophenol quantitation was performed without
P Nagaraja et al.
Farmaco (Societa chimica italiana : 1989), 58(12), 1295-1300 (2003-11-25)
A rapid, simple and sensitive spectrophotometric method for the determination of some sulfa drugs is described. The method is based on the formation of orange yellow colored azo product by the diazotization of sulfonamides, viz., dapsone (DAP), sulfathiazole (SFT), sulfadiazine
[Experimental data for the substantiation of the maximum allowable concentration of meta-aminophenol in the air of work areas].
K L Markarian et al.
Gigiena truda i professional'nye zabolevaniia, (1)(1), 49-50 (1988-01-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.