PHR1338
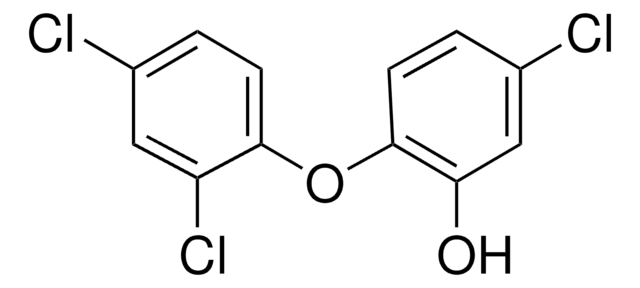
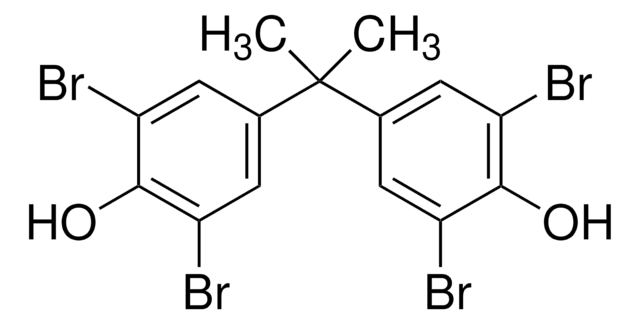
Triclosan
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
Triclosan, 5-Chloro-2-(2,4-dichlorophenoxy)phenol, Irgasan
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C12H7Cl3O2
CAS Number:
Molecular Weight:
289.54
Beilstein:
2057142
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1682206
API family
triclosan
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
Oc1cc(Cl)ccc1Oc2ccc(Cl)cc2Cl
InChI
1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H
InChI key
XEFQLINVKFYRCS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to in-house working standards.
Triclosan is a non-ionic broad spectrum antibacterial and antifungal agent, used in personal care products such as antiseptic soaps, toothpastes, fabrics and plastics.
Triclosan is a non-ionic broad spectrum antibacterial and antifungal agent, used in personal care products such as antiseptic soaps, toothpastes, fabrics and plastics.
애플리케이션
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Triclosan may be used as a pharmaceutical reference standard for the determination of the analyte in waste water samples and food stuffs by various chromatography methods.
A component of cefsulodin-irgasan-novobiocin selection medium for Yersinia, a human pathogen that may contaminate animal-source food products.
생화학적/생리학적 작용
It is an inhibitor of the enoyl-ACP (acyl-carrier protein) reductase component of type II fatty acid synthase (FAS-II) in bacteria and Plasmodium. It also inhibits mammalian fatty acid synthase (FASN), and may have anticarcinogenic activity.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAA9502 in the slot below. This is an example certificate only and may not be the lot that you receive.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Determination of triclosan in foodstuffs
Sanches-Silva A, et al.
Journal of Separation Science, 28(1), 65-72 (2005)
Triclosan: applications and safety
Bhargava HN and Leonard PA
American Journal of Infection Control, 24(3), 209-218 (1996)
Markus K Diener et al.
Lancet (London, England), 384(9938), 142-152 (2014-04-11)
Postoperative surgical site infections are one of the most frequent complications after open abdominal surgery, and triclosan-coated sutures were developed to reduce their occurrence. The aim of the PROUD trial was to obtain reliable data for the effectiveness of triclosan-coated
Siamak P Yazdankhah et al.
Microbial drug resistance (Larchmont, N.Y.), 12(2), 83-90 (2006-08-23)
Triclosan is a widely used biocide that is considered as an effective antimicrobial agent against different microorganisms. It is included in many contemporary consumer and personal health-care products, like oral and dermal products, but also in household items, including plastics
R M Davies et al.
Journal of clinical periodontology, 31(12), 1029-1033 (2004-11-25)
To compare the effectiveness of triclosan/copolymer and fluoride dentifrices in improving plaque control and gingival health. We searched the Cochrane Controlled Trials Register, MEDLINE (1986 to March 2003) and EMBASE (1986 to March 2003). Personal files and the reference lists
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.