S0150000
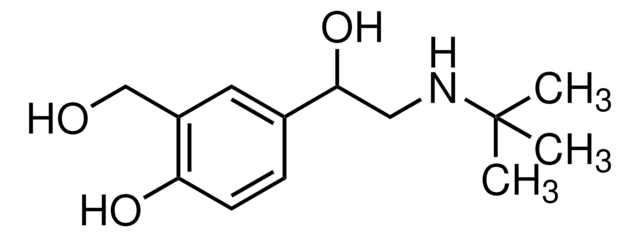
Salbutamol sulfate
European Pharmacopoeia (EP) Reference Standard
동의어(들):
Salbutamol hemisulfate salt, α1-[(tert-Butylamino)methyl]-4-hydroxy-1,3-benzenedimethanol hemisulfate salt, Albuterol sulfate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C13H21NO3 · 0.5H2SO4
CAS Number:
Molecular Weight:
288.35
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
albuterol, salbutamol
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
OS(O)(=O)=O.CC(C)(C)NCC(O)c1ccc(O)c(CO)c1.CC(C)(C)NCC(O)c2ccc(O)c(CO)c2
InChI
1S/2C13H21NO3.H2O4S/c2*1-13(2,3)14-7-12(17)9-4-5-11(16)10(6-9)8-15;1-5(2,3)4/h2*4-6,12,14-17H,7-8H2,1-3H3;(H2,1,2,3,4)
InChI key
BNPSSFBOAGDEEL-UHFFFAOYSA-N
유전자 정보
human ... ADRB2(154)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Salbutamol sulfate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Repr. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
B Padhukasahasram et al.
The pharmacogenomics journal, 14(4), 365-371 (2014-01-15)
Inhaled short-acting beta-agonist (SABA) medication is commonly used in asthma patients to rapidly reverse airway obstruction and improve acute symptoms. We performed a genome-wide association study of SABA medication response using gene-based association tests. A linear mixed model approach was
Marc L Decramer et al.
International journal of chronic obstructive pulmonary disease, 8, 53-64 (2013-02-05)
Inhaled long-acting bronchodilators are the mainstay of pharmacotherapy for chronic obstructive pulmonary disease (COPD). Both the twice-daily long-acting β(2)-adrenoceptor agonists (LABAs) salmeterol and formoterol and the once-daily LABA indacaterol are indicated for use in COPD. This review examines current evidence
Lawrence M Lewis et al.
Chest, 145(1), 53-59 (2013-08-21)
Controversy exists around the incidence and cause of hyperlactatemia during asthma exacerbations. We evaluated the incidence, potential causes, and adverse events of hyperlactatemia in patients with acute asthma exacerbation. This study was a subanalysis of subjects receiving placebo from a
Abebaw M Yohannes et al.
International journal of chronic obstructive pulmonary disease, 8, 117-125 (2013-03-22)
Tiotropium bromide is an anticholinergic agent that has gained worldwide acceptance as a first-line, once daily maintenance therapy for patients with moderate-to-severe chronic obstructive pulmonary disease. The purpose of this review is to synthesize the evidence base in the past
Helgo Magnussen et al.
The New England journal of medicine, 371(14), 1285-1294 (2014-09-10)
Treatment with inhaled glucocorticoids in combination with long-acting bronchodilators is recommended in patients with frequent exacerbations of severe chronic obstructive pulmonary disease (COPD). However, the benefit of inhaled glucocorticoids in addition to two long-acting bronchodilators has not been fully explored.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.