추천 제품
Grade
pharmaceutical primary standard
API family
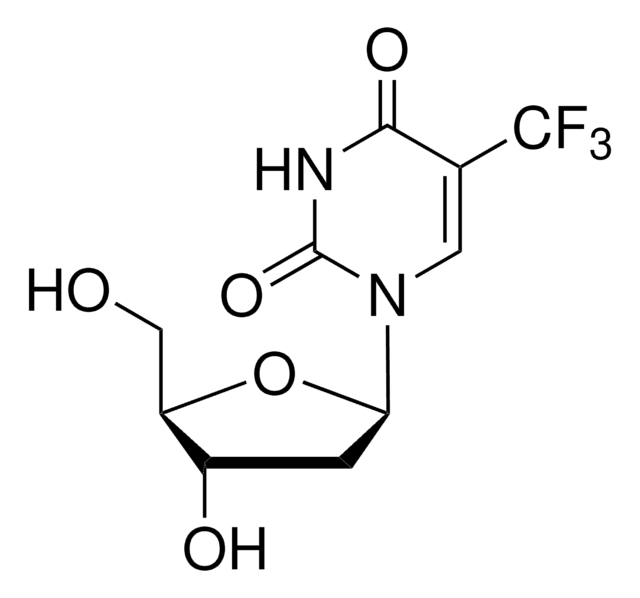
capecitabine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
O[C@H]1[C@@H](O)[C@H](N2C(N=C(NC(OCCCCC)=O)C(F)=C2)=O)O[C@@H]1C
InChI
1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1
InChI key
GAGWJHPBXLXJQN-UORFTKCHSA-N
유전자 정보
human ... TYMS(7298)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Capecitabine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Capecitabine is an anti-cancer drug, a prodrug of doxifluridine, metabolized to 5-fluorouracil at the tumor site. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5′-Deoxy-5-fluorocytidine (5′-DFCR) and 5′-Deoxy-5-fluorouridine (5′-DFUR), to form 5-fluorouracil.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Carc. 1B - Muta. 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Nestor F Esnaola et al.
International journal of radiation oncology, biology, physics, 88(4), 837-844 (2014-03-13)
To evaluate, in a phase 2 study, the safety and efficacy of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer (BRPC or LAPC, respectively). Patients received gemcitabine
Takahiro Yamanashi et al.
Gan to kagaku ryoho. Cancer & chemotherapy, 41(1), 107-112 (2014-01-16)
A 77-year-old man who complained of melena was admitted to our department. Colonoscopy revealed a type 2 tumor in the hepatic flexure of the ascending colon. Biopsy examination revealed a poorly differentiated adenocarcinoma. Abdominal computed tomography(CT)revealed 3 tumors within the
Valentina Sini et al.
Tumori, 99(6), 273e-277e (2014-02-08)
This report describes a case of ab initio metastatic HER2-positive breast cancer in a very young patient. The onset of breast cancer at such a young age is uncommon and could delay the diagnosis with unquestionable impact on the prognosis.
Suna Cokmert et al.
Journal of B.U.ON. : official journal of the Balkan Union of Oncology, 19(1), 75-82 (2014-03-25)
Erythrocyte mean corpuscular volume (MCV) increase has been described in patients treated with capecitabine. In this study, we sought to evaluate the potential association of the erythrocyte MCV increase with tumor response and survival in patients with metastatic colorectal cancer
Moyuru Yamada et al.
Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro-enterology, 111(3), 521-528 (2014-03-07)
A woman in her 60s was referred to our department with advanced rectal cancer and multiple unresectable metastases of the liver and peritoneum. She had been diagnosed with idiopathic thrombocytopenic purpura (ITP) in her 20s, with a platelet count maintained
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.