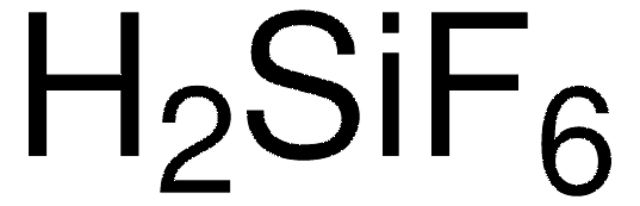모든 사진(1)
About This Item
Linear Formula:
HBF4
CAS Number:
Molecular Weight:
87.81
MDL number:
UNSPSC 코드:
12352106
PubChem Substance ID:
NACRES:
NA.21
추천 제품
일반 설명
Tetrafluoroboric acid is used as a catalyst for the protection and deprotection reactions of various carbohydrates. It participates in the synthesis of 4-sulfonic acid phenyl diazonium tetrafluoroborate, which was required for the preparation of sulfonated graphene (SG).
애플리케이션
Tetrafluoroboric acid solution may be used as a catalyst for the hydration of aromatic haloalkynes to α-halomethyl ketones in the absence of metal catalysts. It may also be used the epoxidized soybean oil (ESBO) ring opening step of fatty acids preparation.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Self-assembling sulfonated graphene/polyaniline nanocomposite paper for high performance supercapacitor.
Fan T, et al.
Synthetic Metals, 199, 79-86 (2015)
Polyols and polyurethanes prepared from epoxidized soybean oil ring-opened by polyhydroxy fatty acids with varying OH numbers.
Chen R, et al.
Journal of Applied Polymer Science, 132(1) (2015)
Tetrafluoroboric acid, an efficient catalyst in carbohydrate protection and deprotection reactions.
Albert R, et al.
Carbohydrate Research, 137, 282-290 (1985)
Metal-free hydration of aromatic haloalkynes to a-halomethyl ketones.
Ye M, et al.
Tetrahedron Letters, 57(45), 4983-4986 (2016)
Richard Ting et al.
Journal of the American Chemical Society, 130(36), 12045-12055 (2008-08-15)
The use of a boronic ester as a captor of aqueous [(18)F]-fluoride has been previously suggested as a means of labeling biomolecules in one step for positron emission tomography (PET) imaging. For this approach to be seriously considered, the [(18)F]-labeled
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.