모든 사진(2)
About This Item
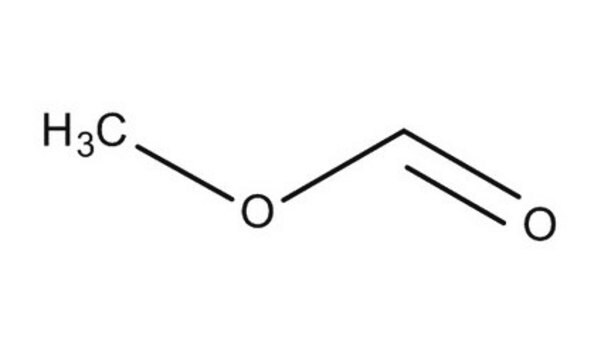
Linear Formula:
HCO2CH3
CAS Number:
Molecular Weight:
60.05
Beilstein:
1734623
EC Number:
MDL number:
UNSPSC 코드:
12352108
PubChem Substance ID:
NACRES:
NA.21
Grade:
spectrophotometric grade
분석:
≥98%
기술:
UV/Vis spectroscopy: suitable
bp:
32-34 °C (lit.)
vapor pressure:
32.81 psi ( 55 °C)
9.21 psi ( 20 °C)
9.21 psi ( 20 °C)
추천 제품
Grade
spectrophotometric grade
Quality Level
vapor density
2.1 (vs air)
vapor pressure
32.81 psi ( 55 °C)
9.21 psi ( 20 °C)
분석
≥98%
양식
liquid
autoignition temp.
842 °F
expl. lim.
23 %
기술
UV/Vis spectroscopy: suitable
불순물
<0.010% water
증발 잔류물
<0.0005%
refractive index
n20/D 1.343 (lit.)
pH
4.0-5.0 (20 °C, 200 g/L)
bp
32-34 °C (lit.)
mp
−100 °C (lit.)
density
0.974 g/mL at 20 °C (lit.)
λ
H2O reference
UV 흡수
λ: 259 nm Amax: 1.00
λ: 260 nm Amax: 0.70
λ: 265 nm Amax: 0.20
λ: 270 nm Amax: 0.04
λ: 310-400 nm Amax: 0.01
SMILES string
[H]C(=O)OC
InChI
1S/C2H4O2/c1-4-2-3/h2H,1H3
InChI key
TZIHFWKZFHZASV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Methyl formate (MF) is one of the most significant intermediates in C1 chemistry. MF has been used to produce methanamide, N, N-dimethylformamide, dimethyl carbonate, methyl glycolate, acetic acid, ethylene glycol, methyl methacrylate, and high-purity CO. Additionally, MF can be used as a solvent for nitrocellulose and cellulose acetate.
애플리케이션
- Bifunctionality of Re Supported on TiO(2) in Driving Methanol Formation in Low-Temperature CO(2) Hydrogenation.: This article explores the catalytic roles of methyl formate in methanol production from CO2 hydrogenation, providing a pathway for sustainable chemical synthesis (Urakawa A et al., 2023).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 1 - STOT SE 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
-2.2 °F - closed cup
Flash Point (°C)
-19 °C - closed cup
Methyl N-Phenyl Carbamate Synthesis from Aniline and Methyl Formate: Carbon Recycling to Chemical Products.
Yalfani MS, et al.
ChemSusChem, 8(3), 443-447 (2015)
Katherine R Phillips et al.
Journal of the American Chemical Society, 135(2), 574-577 (2012-12-28)
Methyl formate is produced from the photo-oxidation of methanol on preoxidized TiO(2)(110). We demonstrate that two consecutive photo-oxidation steps lead to methyl formate using mass spectrometry and scanning tunneling microscopy. The first step in methanol oxidation is formation of methoxy
Barriers to rotation adjacent to double bonds. 3. The carbon-oxygen barrier in formic acid, methyl formate, acetic acid, and methyl acetate. The origin of ester and amide resonance.
Wiberg KB and Laidig KE.
Journal of the American Chemical Society, 109(20), 5935-5943 (1987)
Methyl formate as a carbonylating agent for the catalytic conversion of phenol to methyl phenyl carbonate.
Wu C, et al.
Green Chemistry, 17(3), 1467-1472 (2015)
Microwave spectrum, barrier to internal rotation, and structure of methyl formate.
Curl Jr RF.
J. Chem. Phys. , 30(6), 1529-1536 (1959)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.