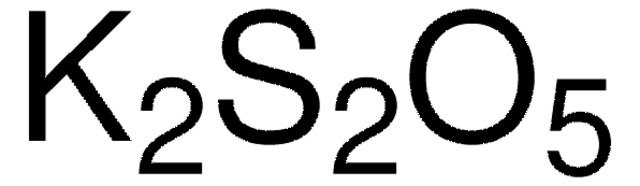모든 사진(2)
About This Item
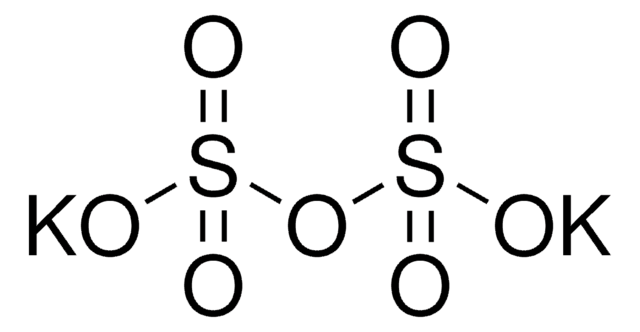
Linear Formula:
K2S2O5
CAS Number:
Molecular Weight:
222.32
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.55
분석:
≥98%
양식:
powder
추천 제품
Quality Level
분석
≥98%
양식
powder
SMILES string
[K+].[K+].[O-]S(=O)S([O-])(=O)=O
InChI
1S/2K.H2O5S2/c;;1-6(2)7(3,4)5/h;;(H,1,2)(H,3,4,5)/q2*+1;/p-2
InChI key
RWPGFSMJFRPDDP-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Potassium disulfite (Potassium metabisulfite, PMB) is an inorganic salt with antimicrobial properties. It is a sulfiting agent that prevents browning of foods. Its genotoxic and cytotoxic effect has been assessed. PMB undergoes hydrolysis to form potassium bisulfite.
애플리케이션
Potassium disulfite has been used in a protocol for the modification of the polydimethylsiloxane (PDMS) polymer surfaces.
It may be used in the following processes:
It may be used in the following processes:
- Chemical etching of poly(vinylidene fluoride) in β-phase (β-PVDF) irradiated films.
- Decolorization during the synthesis of 2-iodopyrimidine derivatives.
- Palladium-catalyzed aminosulfonylation of aryl halides.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Dam. 1
보충제 위험성
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Nanoporous β-PVDF membranes with selectively functionalized pores.
Cuscito O, et al.
Nucl. Instrum. Methods Phys. Res. Sect. B, 265(1), 309-313 (2007)
A palladium-catalyzed reaction of aryl halides, potassium metabisulfite, and hydrazines.
Ye S and Wu J.
Chemical Communications (Cambridge, England), 48(80), 10037-10039 (2012)
Amperometric quantification of sodium metabisulfite in pharmaceutical formulations utilizing tetraruthenated porphyrin film modified electrodes and batch injection analysis.
Quintino MSM, et al.
Talanta, 68(4), 1281-1286 (2006)
Biofunctionalization and self-interaction chromatography in PDMS microchannels.
Deshpande KS, et al.
Biochemical Engineering Journal, 67, 111-119 (2012)
Gábor Vlád et al.
The Journal of organic chemistry, 67(18), 6550-6552 (2002-08-31)
A high-yield synthesis was developed for the preparation of 2,2'-bipyrimidine (1) using the Ullmann coupling of 2-iodopyrimidine. The new procedure was also used for the preparation of 4,4',6,6'-tetramethyl-2,2'-bipyrimidine (2) and 5,5'-dibromo-2,2'-bipyrimidine (3).
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.