모든 사진(3)
About This Item
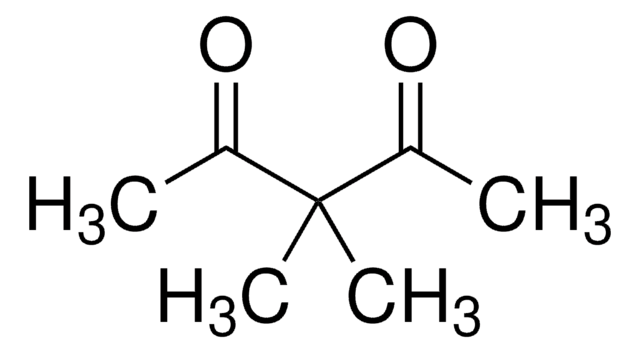
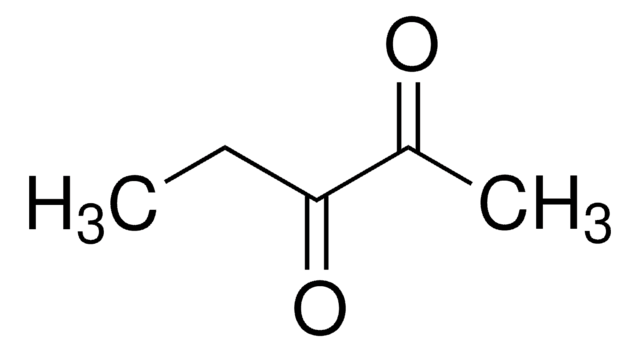
Linear Formula:
CH3COCH2COCH3
CAS Number:
Molecular Weight:
100.12
Beilstein:
741937
EC Number:
MDL number:
UNSPSC 코드:
12352115
eCl@ss:
39021208
PubChem Substance ID:
NACRES:
NA.21
분석:
≥99%
bp:
140.4 °C (lit.)
vapor pressure:
6 mmHg ( 20 °C)
추천 제품
vapor density
3.5 (vs air)
Quality Level
vapor pressure
6 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
autoignition temp.
662 °F
expl. lim.
11.4 %
refractive index
n20/D 1.452 (lit.)
pH
6 (20 °C, 200 g/L)
bp
140.4 °C (lit.)
mp
−23 °C (lit.)
density
0.975 g/mL at 25 °C (lit.)
SMILES string
CC(=O)CC(C)=O
InChI
1S/C5H8O2/c1-4(6)3-5(2)7/h3H2,1-2H3
InChI key
YRKCREAYFQTBPV-UHFFFAOYSA-N
유전자 정보
human ... ACHE(43) , BCHE(590) , CES1(1066)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Acetylacetone (2,4-pentanedione) is an organic compound containing two carbonyl groups and one active methylene group. It is mainly used as an intermediate in the synthesis of various chemical derivatives. It can also be used as a modifier for polyolefins, corrosion inhibitors, and labeling of radiotracers.
애플리케이션
Acetylacetone can be used as:
- A multifunctional ligand in the synthesis and feasible functionalization of gold nanoparticles (AuNPs).
- A reactant to synthesize 9,10-dihydroacridines by reacting with methyl acetoacetate and Morita-Baylis-Hillman acetates.
- A reagent in the synthesis of ZrO2(zirconium dioxide) via hydrolysis of Zr(OC3H7n)4. Acetylacetone controls the hydrolysis and condensation rates of alkoxides and thus, the nucleation and growth rates of oxides.
포장
Packaged in glass bottles
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
95.0 °F - closed cup
Flash Point (°C)
35 °C - closed cup
이미 열람한 고객
Giuliano Alagona et al.
Physical chemistry chemical physics : PCCP, 12(35), 10173-10188 (2010-08-03)
The catalytic effect of explicit water molecules on the keto-enol tautomerism in a system of biological interest (enolpyruvate) has been investigated at the B3LYP/6-31++G** level by exploring the potential energy surface in the presence of 1 or 2 water molecules
Ivelina Georgieva et al.
Journal of molecular modeling, 18(6), 2409-2422 (2011-10-13)
Theoretical and spectroscopic studies of a series of monomeric and dimeric complexes formed through the modification of a zirconium butoxide precursor with acetylacetone and subsequent hydrolysis and/or condensation have been performed by applying DFT/B3LYP/6-31++G(d) and highly accurate RI-ADC(2) methods as
Kyoji Tsuchikama et al.
The Journal of organic chemistry, 76(17), 6981-6989 (2011-06-18)
Bacteria have developed a cell-to-cell communication system, termed quorum sensing (QS), which allows for the population-dependent coordination of their behavior via the exchange of chemical signals. Autoinducer-2 (AI-2), a class of QS signals derived from 4,5-dihydroxy-2,3-pentandione (DPD), has been revealed
Heaweon Park et al.
Inorganic chemistry, 50(23), 11978-11989 (2011-11-01)
A series of high-spin iron(II) β-diketonato complexes have been prepared and characterized with the intent of modeling the substrate-bound form of the enzyme acetylacetone dioxygenase (Dke1). The Dke1 active site features an Fe(II) center coordinated by three histidine residues in
Rolando R Lozada-García et al.
Physical chemistry chemical physics : PCCP, 14(10), 3450-3459 (2012-02-07)
The photochemistry of the chelated enol form of acetylacetone (AcAc) was investigated by UV excitation of the S(2) state at 266 nm in parahydrogen matrices, complemented by experiments in neon and normal hydrogen matrices. Infrared (IR) spectroscopy, combined with theoretical
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.