06863
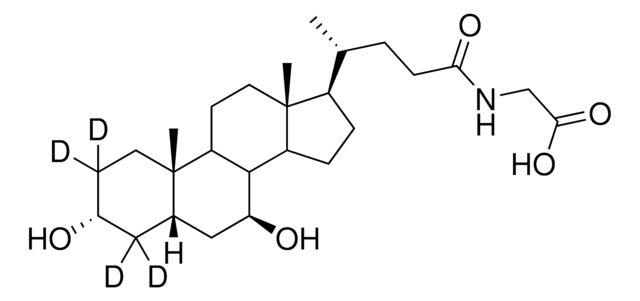
Glycoursodeoxycholic acid
≥96.0% (TLC)
동의어(들):
N-(3α,7β-Dihydroxy-5β-cholan-24-oyl)glycine, N-[(3α,5β,7β)-3,7-Dihydroxy-24-oxocholan-24-yl]glycine, GUDCA, Glycylursodeoxycholic acid, Ursodeoxycholylglycine
About This Item
추천 제품
생물학적 소스
synthetic
Quality Level
분석
≥96.0% (TLC)
양식
powder
작용기
carboxylic acid
SMILES string
[H][C@@]12[C@]([C@](CC[C@@H](O)C3)(C)[C@]3([H])C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC(NCC(O)=O)=O
[H][C@@]12[C@]([C@](CC[C@@H](O)C3)(C)[C@]3([H])C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC(NCC(O)=O)=O
InChI
1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21+,24+,25+,26-/m1/s1
InChI key
GHCZAUBVMUEKKP-XROMFQGDSA-N
애플리케이션
- Glycoursodeoxycholic Acid Alleviates Arterial Thrombosis via Suppressing Diacylglycerol Kinases Activity in Platelet.: Highlights the therapeutic potential of Glycoursodeoxycholic acid in alleviating arterial thrombosis by inhibiting diacylglycerol kinase activity in platelets (Yang et al., 2024).
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
프로토콜
Investigate bile acid roles in gut hormone profiles and glycemic control, vital for clinical labs exploring potential mechanisms.
관련 콘텐츠
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.