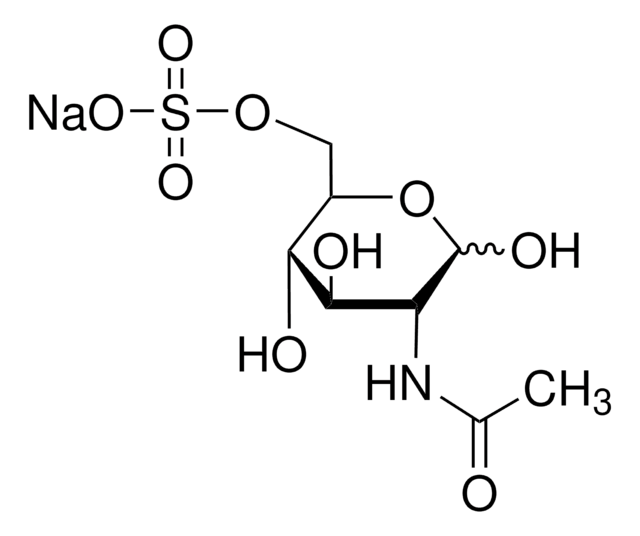
모든 사진(1)
About This Item
실험식(Hill 표기법):
C6H13NO8S
CAS Number:
Molecular Weight:
259.23
MDL number:
UNSPSC 코드:
12352201
PubChem Substance ID:
NACRES:
NA.25
추천 제품
Quality Level
분석
≥98.0% (TLC)
형태
powder
광학 활성
[α]/D 55.0±2.0
기술
thin layer chromatography (TLC): suitable
저장 온도
−20°C
SMILES string
N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1OS(O)(=O)=O
InChI
1S/C6H13NO8S/c7-3-5(15-16(11,12)13)4(9)2(1-8)14-6(3)10/h2-6,8-10H,1,7H2,(H,11,12,13)/t2-,3-,4-,5-,6?/m1/s1
InChI key
UZUBNIPDAIVWIE-IVMDWMLBSA-N
애플리케이션
D-Glucosamine 3-sulfate (GlcN-3S) may be used as a reference in analytical analysis of the components of heparin sulfate.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
A S Edge et al.
The Journal of biological chemistry, 265(26), 15874-15881 (1990-09-15)
Fragmentation of the heparan sulfate chains from bovine glomerular basement membrane (GBM) by hydrazine/nitrous acid treatment followed by NaB3H4-reduction yielded a mixture of six sulfated disaccharides containing D-glucuronic (GlcUA) or L-iduronic acid (IdUA) and terminating in 2,5-anhydro[3H]mannitol (AnManH2), in addition
A Naggi et al.
Carbohydrate research, 336(4), 283-290 (2001-12-01)
In the framework of a project aimed at generating heparin-like sulfation patterns and biological activities in biotechnological glycosaminoglycans, different approaches have been considered for simulating the alpha(1-->4)-linked 2-O-sulfated L-iduronic acid (IdoA2SO(3))-->N,6-O-sulfated D-glucosamine (GlcNSO(3)6SO(3)) disaccharide sequences prevalent in mammalian heparins. Since
H G Garg et al.
Biochemical and biophysical research communications, 224(2), 468-473 (1996-07-16)
Heparin macromolecules inhibit vascular remodeling associated with hypoxic pulmonary hypertension. Heparin's antiproliferative effect on smooth muscle cells, based on studies of synthetic pentasaccharide fragments, has been attributed to 3-O-sulfate on the internal glucosamine. To determine the role of 3-O-sulfation in
U Lindahl et al.
Proceedings of the National Academy of Sciences of the United States of America, 77(11), 6551-6555 (1980-11-01)
An octasaccharide with high affinity for antithrombin was isolated after partial deaminative cleavage of heparin with nitrous acid. After conversion of the 2,5-anhydro-D-mannose end group to anhydro[1-3H]mannitol, labeled pentasaccharide was released from the octasaccharide by periodate-alkali treatment. Incubation of the
Alessandro Parra et al.
Glycobiology, 22(2), 248-257 (2011-09-22)
Glycosaminoglycans were extracted from both young rabbit growth plate (GRP) and articular (ART) cartilage tissues and enzymatically treated to selectively eliminate chondroitin sulfates and hyaluronic acid. The procedure avoided any fractionation step that could enrich the extract with over- or
문서
Glycosaminoglycans are large linear polysaccharides constructed of repeating disaccharide units.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.