모든 사진(1)
About This Item
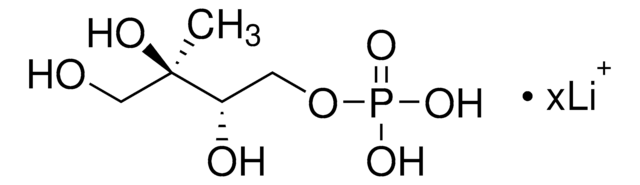
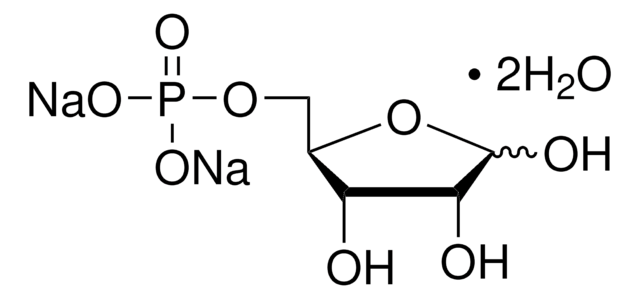
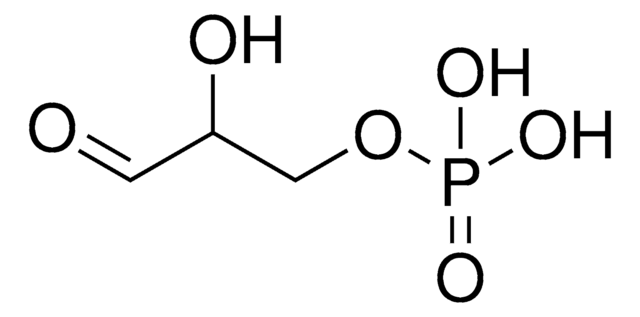
실험식(Hill 표기법):
C5H11O7P · xNa+
CAS Number:
Molecular Weight:
214.11 (free acid basis)
Beilstein:
8367371
MDL number:
UNSPSC 코드:
12352201
NACRES:
NA.25
추천 제품
Quality Level
분석
≥99.0% (TLC)
양식
powder
광학 활성
[α]/D 37.0±3.0°, c = 0.1 in 0.1 M HCl
색상
white
저장 온도
−20°C
SMILES string
[P](=O)(OC[C@@H](O)[C@H](O)C(=O)C)(O)O
InChI
1S/C5H11O7P/c1-3(6)5(8)4(7)2-12-13(9,10)11/h4-5,7-8H,2H2,1H3,(H2,9,10,11)/t4-,5-/m1/s1
InChI key
AJPADPZSRRUGHI-RFZPGFLSSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
1-Deoxy-D-xylulose-5-phosphate is used as a substrate for the identification, differentiation and characterization of procaryotic 1-Deoxy-D-xylulose-5-phosphate reductoisomerase(s) (Dxr) which catalyze the first committed step of the nonmevalonate pathway (NMP) for isoprenoid biosynthesis.
생화학적/생리학적 작용
Metabolite of the non-mevalonate pathway, generally found in prokaryotes, as precursor to isoprenoids as well asnon-isoprenoids like vitamins. As this pathway is not present in humans, it is of interest for the development of bacterium-specific drugs in the search for treatments of infectious diseases.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
W Eisenreich et al.
Chemistry & biology, 5(9), R221-R233 (1998-09-30)
Recent studies have uncovered the existence of an alternative, non-mevalonate pathway for the formation of isopentenyl pyrophosphate and dimethylallyl pyrophosphate, the two building blocks of terpene biosynthesis.
Y Boucher et al.
Molecular microbiology, 37(4), 703-716 (2000-09-06)
Lateral gene transfer (LGT) is a major force in microbial genome evolution. Here, we present an overview of lateral transfers affecting genes involved in isopentenyl diphosphate (IPP) synthesis. Two alternative metabolic pathways can synthesize this universal precursor of isoprenoids, the
B M Lange et al.
Proceedings of the National Academy of Sciences of the United States of America, 95(5), 2100-2104 (1998-04-16)
Isopentenyl diphosphate, the common precursor of all isoprenoids, has been widely assumed to be synthesized by the acetate/mevalonate pathway in all organisms. However, based on in vivo feeding experiments, isopentenyl diphosphate formation in several eubacteria, a green alga, and plant
H Jomaa et al.
Science (New York, N.Y.), 285(5433), 1573-1576 (1999-09-08)
A mevalonate-independent pathway of isoprenoid biosynthesis present in Plasmodium falciparum was shown to represent an effective target for chemotherapy of malaria. This pathway includes 1-deoxy-D-xylulose 5-phosphate (DOXP) as a key metabolite. The presence of two genes encoding the enzymes DOXP
G A Sprenger et al.
Proceedings of the National Academy of Sciences of the United States of America, 94(24), 12857-12862 (1997-12-16)
In Escherichia coli, 1-deoxy-D-xylulose (or its 5-phosphate, DXP) is the biosynthetic precursor to isopentenyl diphosphate [Broers, S. T. J. (1994) Dissertation (Eidgenössische Technische Hochschule, Zürich)], thiamin, and pyridoxol [Himmeldirk, K., Kennedy, I. A., Hill, R. E., Sayer, B. G. &
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.