69587
2′-(4-Methylumbelliferyl)-α-D-N-acetylneuraminic acid sodium salt hydrate
BioReagent, suitable for fluorescence, ≥96.5% (HPLC)
동의어(들):
4-MUNANA, 4-Methylumbelliferyl-N-acetyl-α-D-neuraminic acid sodium salt hydrate, 4-Methylumbelliferyl-N-acetyl-α-D-neuraminic acid sodium salt hydrate
About This Item
추천 제품
제품 라인
BioReagent
분석
≥96.5% (HPLC)
양식
powder
불순물
≤0.5% free 4-methylumbelliferone
≤10% water
solubility
H2O: 50 mg/mL, clear, very slightly yellow
형광
λex 315 nm; λem 374 nm (pH.7.0)
λex 365 nm; λem 445 nm (after cleavage by neuraminidase)
적합성
suitable for fluorescence
저장 온도
−20°C
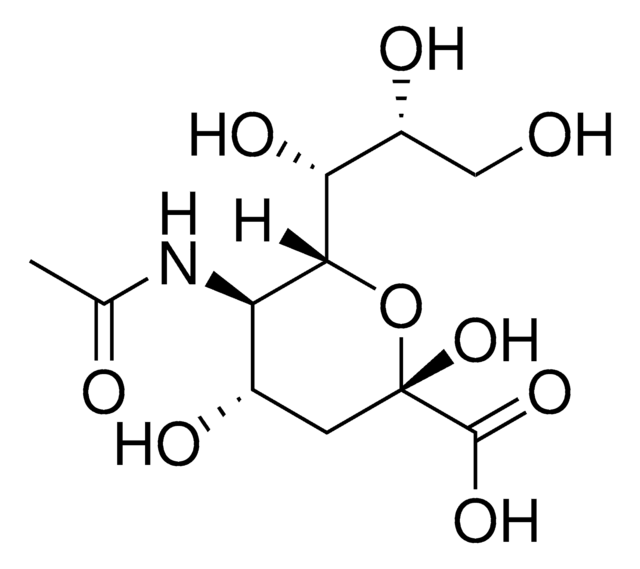
SMILES string
O.[H]C(O)(CO)[C@]([H])(O)[C@@H]1OC(C[C@H](O)[C@H]1NC(C)=O)(Oc2ccc3C(C)=CC(=O)Oc3c2)C(=O)O[Na]
InChI
1S/C21H25NO11.Na.H2O/c1-9-5-16(27)31-15-6-11(3-4-12(9)15)32-21(20(29)30)7-13(25)17(22-10(2)24)19(33-21)18(28)14(26)8-23;;/h3-6,13-14,17-19,23,25-26,28H,7-8H2,1-2H3,(H,22,24)(H,29,30);;1H2/q;+1;/p-1/t13-,14?,17+,18?,19+,21+;;/m0../s1
InChI key
NSQMRVBWXQQIKF-NVRWCLOTSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
Understand sialic acid structure, function, signaling, and modifications. Easily find products for sialic acid research.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.