추천 제품
product name
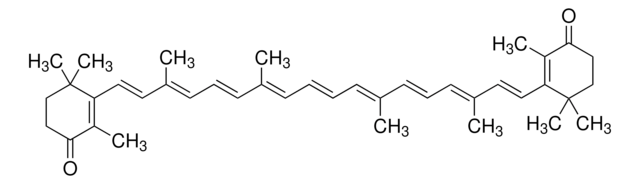
Echinenone, ≥95.0% (HPLC)
Quality Level
분석
≥95.0% (HPLC)
형태
solid
λ
in hexane (with 2% dichloromethane)
UV 흡수
λ: 459 nm±5 nm Amax
저장 온도
−20°C
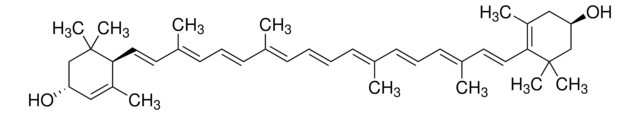
SMILES string
CC(/C=C/C1=C(C)C(CCC1(C)C)=O)=C\C=C\C(C)=C\C=C\C=C(C)\C=C\C=C(\C=C\C2=C(C)CCCC2(C)C)C
InChI
1S/C40H54O/c1-30(18-13-20-32(3)23-25-36-34(5)22-15-28-39(36,7)8)16-11-12-17-31(2)19-14-21-33(4)24-26-37-35(6)38(41)27-29-40(37,9)10/h11-14,16-21,23-26H,15,22,27-29H2,1-10H3/b12-11+,18-13+,19-14+,25-23+,26-24+,30-16+,31-17+,32-20+,33-21+
InChI key
QXNWZXMBUKUYMD-QQGJMDNJSA-N
일반 설명
Echinenone is a carotenoid with a conjugated carbonyl group. Echinenone (4-keto-β-carotene) is a monoketo compound, is an intermediate between β-carotene and canthaxanthin in animals. It is one of the major carotenoids of Anabaena sp., and also present in Micrococcus roseus. It is located in the thylakoid membrane of Anabaena. The conversion of β-carotene to echinenone is catalysed by CrtO, a β-carotene ketolase.
애플리케이션
Echinenone may be used as an external standard for the extraction of carotenoid from plasma samples. It may also been used as an internal standard, added to samples for extraction and quantification.
생화학적/생리학적 작용
Echinenone binds to glocobacter rhodopsin and functions as a light harvesting antenna in Gloeobacter violaceous.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Que Chen et al.
Photochemistry and photobiology, 93(3), 772-781 (2017-05-14)
Proteorhodopsins are light-driven proton pumps that occur widespread in Nature, where they function predominantly in environments with high incident irradiance. Their maximal absorbance is usually in the blue range, but can be extended into the (far)red range of the electromagnetic
Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance
Pialoux V, et al.
European Journal of Clinical Nutrition, 60(12), 1345-1345 (2006)
Paula Mapelli-Brahm et al.
Food chemistry, 300, 125232-125232 (2019-07-29)
The mechanisms of main tomato carotenes (phytoene, phytofluene, lycopene and β-carotene) intestinal absorption are still only partly understood. We thus compared carotene bioavailability in mice after gavage with carotene-rich oil-in-water emulsions. We also determined each carotene absorption profile along the
Fernando Muzzopappa et al.
Nature plants, 5(10), 1076-1086 (2019-09-19)
The photoactive orange carotenoid protein (OCP) is a blue-light intensity sensor involved in cyanobacterial photoprotection. Three OCP families co-exist (OCPX, OCP1 and OCP2), having originated from the fusion of ancestral domain genes. Here, we report the characterization of an OCPX
Veno Jaša Grujić et al.
Plants (Basel, Switzerland), 11(8) (2022-04-22)
H. pluvialis is a unicellular freshwater alga containing many bioactive compounds, especially carotenoids, which are the strongest antioxidants among the pigments. This study evaluates the composition and content of carotenoids and other pigments in both stages of algae life cycle
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.