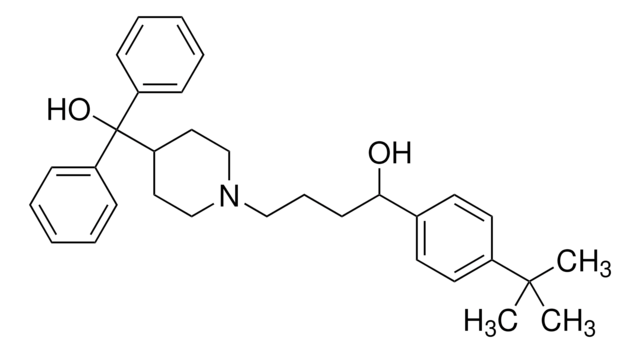
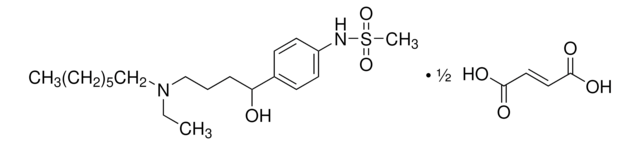
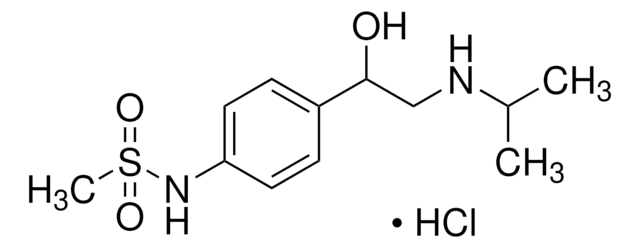
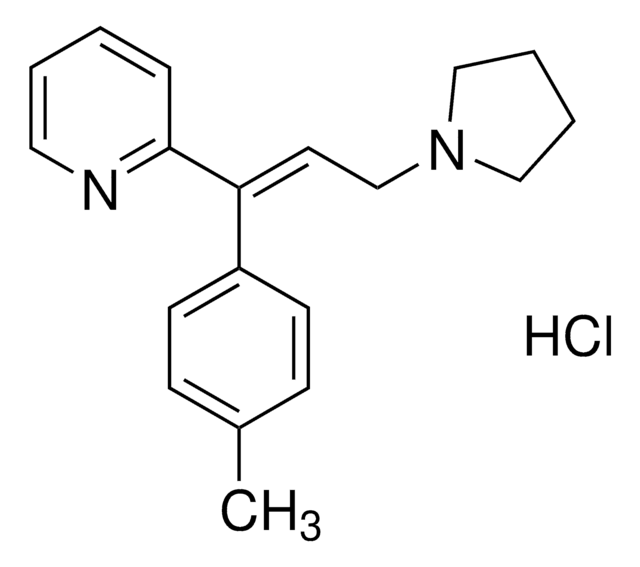
추천 제품
Quality Level
분석
≥98% (HPLC)
형태
powder
저장 조건
desiccated
protect from light
solubility
DMSO: >20 mg/mL
주관자
Johnson & Johnson
저장 온도
2-8°C
SMILES string
COc1ccc(CCN2CCC(CC2)Nc3nc4ccccc4n3Cc5ccc(F)cc5)cc1
InChI
1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31)
InChI key
GXDALQBWZGODGZ-UHFFFAOYSA-N
유전자 정보
human ... HRH1(3269)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
Astermizole is a potent hERG potassium channel blocker (IC50 of 0.9 nM) and may used as a pharmacological chaperone to correct folding defects and restore protein function for some mutated forms of hERG channels. It has also been studied for treatment of malaria, hERG and hEAG channel function in cancer and as a second generation antihistamine H-1 antagonist.
특징 및 장점
This compound is featured on the Potassium Channels page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Johnson & Johnson. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
K A Rao et al.
Mayo Clinic proceedings, 69(6), 589-593 (1994-06-01)
An overdose of astemizole predisposes the myocardium to ventricular dysrhythmias, including torsades de pointes. Herein we describe a case of astemizole-induced torsades de pointes ventricular tachycardia and also review previous case reports in the literature. All the patients were young
Kiem Vu et al.
Medical mycology, 48(2), 255-262 (2009-07-03)
Cryptococcus neoformans is the leading cause of fungal meningitis, a life-threatening infection that occurs predominately in immuocompromised patients. Current drug therapies are limited to amphotericin B, flucytosine and the azoles since the echinocandins have no demonstrated activity against yeast like
Fumimasa Nomura et al.
Journal of nanobiotechnology, 9, 39-39 (2011-09-21)
Conventional in vitro approach using human ether-a-go-go related gene (hERG) assay has been considered worldwide as the first screening assay for cardiac repolarization safety. However, it does not always oredict the potential QT prolongation risk or pro-arrhythmic risk correctly. For
Tjøstil Vlaar et al.
Angewandte Chemie (International ed. in English), 51(52), 13058-13061 (2012-11-20)
O(2) in, H(2)O out: Various diamines and related bisnucleophiles readily undergo oxidative isocyanide insertion with Pd(OAc)(2) (1 mol %) as the catalyst and O(2) as the terminal oxidant to give a diverse array of medicinally relevant N heterocycles. The utility
Chitalu C Musonda et al.
Bioorganic & medicinal chemistry letters, 19(2), 481-484 (2008-12-05)
A dual activity, conjugated approach has been taken to form hybrid molecules of two known antimalarial drugs, chloroquine (CQ) and the non-sedating H1 antagonist astemizole. A variety of linkers were investigated to conjugate the two agents into one molecule. Compounds
문서
We offer many products related to potassium channels for your research needs.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.