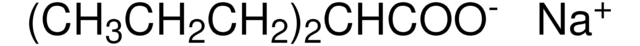A5513
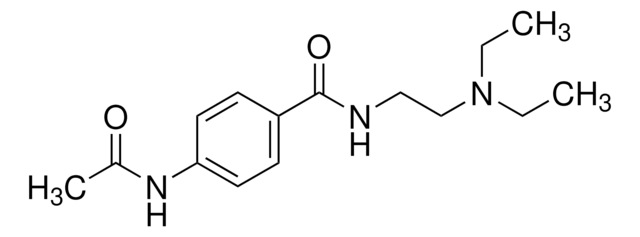
N-Acetylprocainamide hydrochloride
≥99% (HPLC), powder
동의어(들):
N-Acetylnovocainamide hydrochloride, Acecainide hydrochloride, NAPA
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
4-(CH3CONH)C6H4CONHCH2CH2N(C2H5)2·HCl
CAS Number:
Molecular Weight:
313.82
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥99% (HPLC)
양식
powder
mp
184-186 °C (lit.)
solubility
H2O: 50 mg/mL
저장 온도
−20°C
SMILES string
Cl[H].CCN(CC)CCNC(=O)c1ccc(NC(C)=O)cc1
InChI
1S/C15H23N3O2.ClH/c1-4-18(5-2)11-10-16-15(20)13-6-8-14(9-7-13)17-12(3)19;/h6-9H,4-5,10-11H2,1-3H3,(H,16,20)(H,17,19);1H
InChI key
IYEWBJUCJHKLHD-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
N-Acetylprocainamide hydrochloride may be used:
- as an internal standard for spiking plasma samples for ultra-high-pressure liquid chromatography coupled with a diode array detector (UHPLC-DAD) analysis
- to test its relaxant effect on tracheal smooth muscle tissue preparations
- in preparation of complexes with N-acetyl-L-tyrosine methyl ester and N-acetyl-L-phenylalanine methyl ester for studying intermolecular interactions using nuclear magnetic resonance (NMR) spectroscopy studies
N-Acetylprocainamide hydrochloride is a class III antiarrhythmic compound. N-Acetylprocainamide hydrochloride has been used in a study to determine the disposition of procainamide and N-acetylprocainamide in protein-calorie malnutrition. N-Acetylprocainamide hydrochloride has also been used to study pharmacokinetics of procainamide and N-acetylprocainamide in rats.
생화학적/생리학적 작용
N-acetyltransferase II in liver catalyzes the conversion of procainamide to N-acetylprocainamide (NAPA).
Class III antiarrhythmic acting on potassium currents.
Class III antiarrhythmic. Increases the duration of the action potential by decreasing the delayed outward potassium current, slightly decreasing the calcium current, and slightly depressing the inward rectifier potassium current. This is the active metabolite of procainamide that does not induce systemic lupus erythematosus.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
D Jung et al.
Drug metabolism and disposition: the biological fate of chemicals, 13(3), 359-363 (1985-05-01)
The influence of dietary protein deficiency on the disposition of procainamide (PA) and its major metabolite, N-acetylprocainamide (NAPA) was investigated in male Sprague-Dawley rats fed for 4 weeks on a 23 (control) or a 5% (low) protein diet ad libitum.
The evidence for complex formation between N-acetyl-l-tyrosine methyl ester and N-acetylprocainamide hydrochloride using NMR spectroscopy
Janik A, et al.
Structural Chemistry, 20(4), 699-707 (2009)
B L Kamath et al.
Journal of pharmaceutical sciences, 70(3), 299-302 (1981-03-01)
The pharmacokinetics of distribution and elimination of procainamide and its major metabolite, N-actylprocainamide, were studied in rats. Eight rats were selected randomly, and each received intravenously 14C-labeled procainamide hydrochloride (75 mg/kg) or 14C-labeled N-acetylprocainamide hydrochloride (86 mg/kg) according to a
Anusha Balla et al.
Pharmaceutics, 10(2) (2018-03-31)
A simple, sensitive, and reliable reversed-phase, Ultra-High-Pressure Liquid Chromatography (UHPLC) coupled with a Diode Array Detector (DAD) method for the simultaneous determination of Procainamide (PA) and its major metabolite, N-acetylprocainamide (NAPA), in rat plasma was developed and validated. A simple
K Okumura et al.
Clinical pharmacology and therapeutics, 61(5), 509-517 (1997-05-01)
We studied the genotypes of polymorphic N-acetyltransferase (NAT2) in 145 Japanese subjects by the polymerase chain reaction-restriction fragment length polymorphism method. The rapid-type NAT2*4 was expressed at a higher frequency (68.6%) than the slow-type genes with specific point mutations (NAT2*6A
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.