C5249
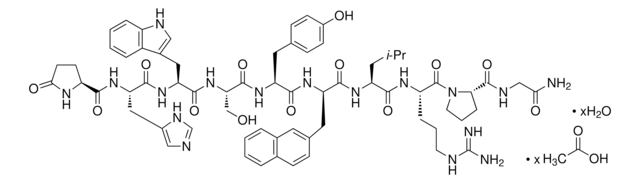
Cetrorelix acetate
≥98% (HPLC)
동의어(들):
Cetrotide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N5-(aminocarbonyl)-D-ornithyl-L-leucyl-L-arginyl-L-prolyl-D-alaninamide acetic acid salt, NS-75A, SB-75
About This Item
추천 제품
Quality Level
분석
≥98% (HPLC)
형태
solid
solubility
methanol: 10%
주관자
Merck & Co., Inc., Kenilworth, NJ, U.S.
저장 온도
−20°C
SMILES string
CC(O)=O.CC(C)C[C@H](NC(=O)[C@@H](CCCNC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc2cccnc2)NC(=O)[C@@H](Cc3ccc(Cl)cc3)NC(=O)[C@@H](Cc4ccc5ccccc5c4)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N6CCC[C@H]6C(=O)N[C@H](C)C(N)=O
InChI
1S/C70H92ClN17O14.C2H4O2/c1-39(2)31-52(61(94)82-51(15-9-28-77-69(73)74)68(101)88-30-10-16-58(88)67(100)79-40(3)59(72)92)83-60(93)50(14-8-29-78-70(75)102)81-63(96)54(34-43-20-25-49(91)26-21-43)86-66(99)57(38-89)87-65(98)56(36-45-11-7-27-76-37-45)85-64(97)55(33-42-18-23-48(71)24-19-42)84-62(95)53(80-41(4)90)35-44-17-22-46-12-5-6-13-47(46)32-44;1-2(3)4/h5-7,11-13,17-27,32,37,39-40,50-58,89,91H,8-10,14-16,28-31,33-36,38H2,1-4H3,(H2,72,92)(H,79,100)(H,80,90)(H,81,96)(H,82,94)(H,83,93)(H,84,95)(H,85,97)(H,86,99)(H,87,98)(H4,73,74,77)(H3,75,78,102);1H3,(H,3,4)/t40-,50-,51+,52+,53-,54+,55-,56-,57+,58+;/m1./s1
InChI key
KFEFLCOCAHJBEA-ANRVCLKPSA-N
유전자 정보
human ... GNRHR(2798)
애플리케이션
생화학적/생리학적 작용
특징 및 장점
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.