추천 제품
product name
Differentiated HepaRG™ Cells Cryopreserved,
생물학적 소스
(Liver tumor of female patient diagnosed with hepatocarcinoma and hepatitis C)
배송 상태
dry ice
저장 온도
−196°C
일반 설명
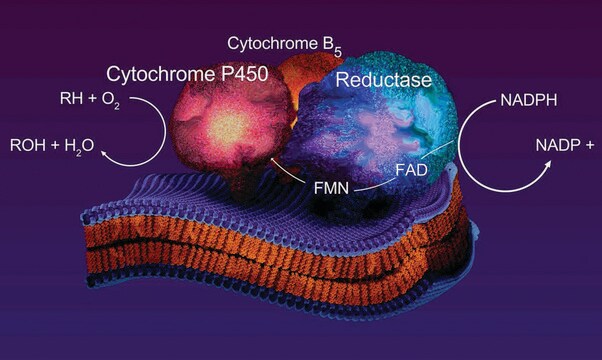
HepaRG is a human hepatoma cell line originally derived from a female patient diagnosed with hepatocarcinoma and hepatitis C. The cells possess a pseudodiploid karyotype and have been characterized as an oval ductular bipotent hepatic cell line that has the ability to differentiate into both biliary and hepatocyte lineages in the presence of DMSO. HepaRG cells are useful as an alternative to human primary cells as they retain the major functional activities of these cells. HepaRG cells express functional levels of the major xenobiotic sensors (PXR, CAR and AhR), drug transporters, phase I and II drug metabolizing enzymes as well as key hepatic transcription factors involved in stress response pathways. HepaRG cells are fully differentiated and ready to use upon receipt.
애플리케이션
See user guide for detailed protocols.
특징 및 장점
HepaRG cells are useful for many in vitro ADME applications, including drug metabolism,reactive metabolite formation, clearance, CYP inhibition and induction assays. They are also suitable for a variety of in vitro toxicity assays, including general hepatotoxicity, cholestasis,and viral infection. HepaRG cells have recently been grown in bioreactors, used for the generation of hepatic spheroids and been implanted into animals to produce humanized livers. The potential range of applications for HepaRG cells continues to expand at a rapid pace.
법적 정보
Use of this product is subject to one or more license agreements.
HepaRG cells are patented and their use is strictly limited; consider the cells as a single use, disposable product that must be destroyed upon conclusion of a study or experiment. Propagating, reproducing, cloning, subcloning or any other use of the cells following the conclusion of a study is prohibited. Use of the cells to produce or manufacture commercial products for general sale or for use in the manufacture of products intended for general sale is prohibited. Transfer of the cells to anyone not employed within the same organization, whether for financial benefit or not, is prohibited. If you are unwilling to accept the terms of this LIMITED USE LICENSE, do not ORDER or use them, and immediately return the cells for credit. Violators of this Limited Use License will be prosecuted to the fullest extent of the law.
HepaRG cells are patented and their use is strictly limited; consider the cells as a single use, disposable product that must be destroyed upon conclusion of a study or experiment. Propagating, reproducing, cloning, subcloning or any other use of the cells following the conclusion of a study is prohibited. Use of the cells to produce or manufacture commercial products for general sale or for use in the manufacture of products intended for general sale is prohibited. Transfer of the cells to anyone not employed within the same organization, whether for financial benefit or not, is prohibited. If you are unwilling to accept the terms of this LIMITED USE LICENSE, do not ORDER or use them, and immediately return the cells for credit. Violators of this Limited Use License will be prosecuted to the fullest extent of the law.
HepaRG is a trademark of BioPredic International company
면책조항
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
보충제
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Repr. 1A
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Catherine C Bell et al.
Drug metabolism and disposition: the biological fate of chemicals, 45(4), 419-429 (2017-02-01)
Reliable and versatile hepatic in vitro systems for the prediction of drug pharmacokinetics and toxicity are essential constituents of preclinical safety assessment pipelines for new medicines. Here, we compared three emerging cell systems-hepatocytes derived from induced pluripotent stem cells, HepaRG
Maxime Demazeau et al.
International journal of pharmaceutics, 524(1-2), 268-278 (2017-04-04)
In this study, we evaluated cationic liposomes prepared from diether-NH
Anika Mann et al.
Human cell, 30(4), 267-278 (2017-05-21)
HepaRG cells are widely used as an in vitro model to assess drug-induced hepatotoxicity. However, only few studies exist so far regarding their suitability to detect the effects of drugs requiring a preceding activation via the cytochrome P450 (CYP) system.
Yi Ni et al.
Methods in molecular biology (Clifton, N.J.), 1540, 15-25 (2016-12-16)
Investigations of virus-host interactions rely on suitable in vitro cell culture systems that efficiently support virus infection. Such systems should ideally provide conditions that resemble those of natural host cells, e.g., the cell-type specific signaling and metabolic pathways. For HBV
Lindsey M Ott et al.
SLAS discovery : advancing life sciences R & D, 22(5), 614-625 (2017-03-28)
Drug-induced liver injury (DILI) and drug-drug interactions (DDIs) are concerns when developing safe and efficacious compounds. We have developed an automated multiplex assay to detect hepatotoxicity (i.e., ATP depletion) and metabolism (i.e., cytochrome P450 1A [CYP1A] and cytochrome P450 3A4
문서
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.