I100
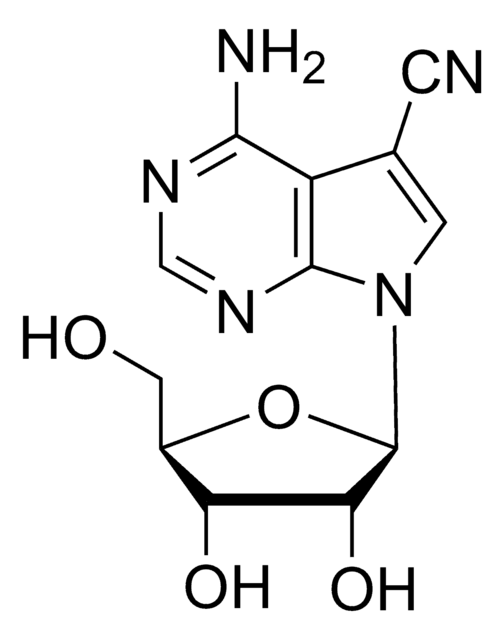
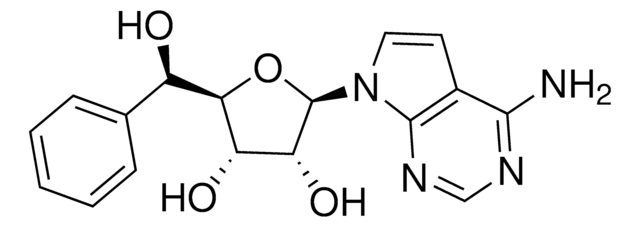
5-Iodotubercidin
≥85%, solid
동의어(들):
4-Amino-5-iodo-7-(β-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine, 5-Iodotubericidin, 7-Iodo-7-deazaadenosine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C11H13IN4O4
CAS Number:
Molecular Weight:
392.15
MDL number:
UNSPSC 코드:
12352202
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥85%
형태
solid
저장 온도
2-8°C
SMILES string
Nc1ncnc2n(cc(I)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1
InChI key
WHSIXKUPQCKWBY-IOSLPCCCSA-N
일반 설명
5-Iodotubercidin is a purine and modulates cellular adenosine levels by inhibiting the functionality of adenosine kinase. It is an activator for p53 and favors tumor suppression and may serve as a potential chemotherapeutic agent. 5-Iodotubercidin mediated blockade of adenosine to adenosine monophosphate (AMP) conversion results in alleviating antinociceptive effect of AMP. 5-Iodotubercidin is under clinical trial testing for treating epilepsy.
solubility: 10 mg/mL in DMSO
애플리케이션
5-Iodotubercidin has been used for the inhibition of retinoblastoma cells, astroglial cultures and for the inhibition of adenosine kinase in human umbilical vein endothelial cells (HUVECs).
생화학적/생리학적 작용
Potent inhibitor of adenosine uptake into brain, and of adenosine kinase and subsequent metabolism of adenine nucleotides. In cultured rat hepatocytes, 5-iodotubercidin inhibits both acetyl-CoA carboxylase and de novo synthesis of fatty acids and cholesterol.
5-Iodotubercidin increases fatty acid oxidation activity and glycogen synthesis in hepatocytes. 5-Iodotubercidin is also a potent inhibitor of adenosine uptake into brain.
주의사항
Solutions may be stored frozen. Use promptly when thawed and protect from exposure to light.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Understanding the basic mechanisms underlying seizures in mesial temporal lobe epilepsy and possible therapeutic targets: a review
O'dell CM, et al.
Journal of Neuroscience Research, 90(5), 913-924 (2012)
Ecto-5?-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits
Sowa NA, et al.
The Journal of Neuroscience, 30(6), 2235-2244 (2010)
Opposite modulation of astroglial proliferation by adenosine 5?-O-(2-thio)-diphosphate and 2-methylthioadenosine-5?-diphosphate: mechanisms involved
Quintas C, et al.
Neuroscience, 182(8), 32-42 (2011)
Retinoblastoma cells are inhibited by aminoimidazole carboxamide ribonucleotide (AICAR) partially through activation of AMP-dependent kinase
Theodoropoulou S, et al.
Faseb Journal, 24(8), 2620-2630 (2010)
D Massillon et al.
The Biochemical journal, 299 ( Pt 1), 123-128 (1994-04-01)
Addition of micromolar concentrations of the adenosine derivative 5-iodotubercidin (Itu) initiates glycogen synthesis in isolated hepatocytes by causing inactivation of phosphorylase and activation of glycogen synthase [Flückiger-Isler and Walter (1993) Biochem. J. 292, 85-91]. We report here that Itu also
문서
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.