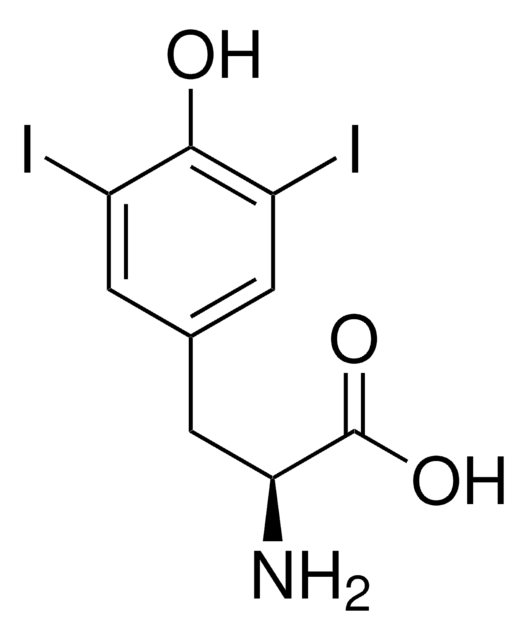
모든 사진(1)
About This Item
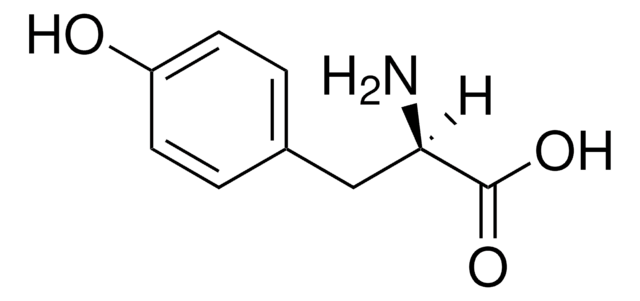
Linear Formula:
IC6H3-4-(OH)CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
307.09
Beilstein:
2941266
EC Number:
MDL number:
UNSPSC 코드:
12352200
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.32
추천 제품
Quality Level
불순물
~5% tyrosine
mp
210 °C (dec.) (lit.)
solubility
dilute aqueous acid: soluble
저장 온도
−20°C
SMILES string
N[C@@H](Cc1ccc(O)c(I)c1)C(O)=O
InChI
1S/C9H10INO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)/t7-/m0/s1
InChI key
UQTZMGFTRHFAAM-ZETCQYMHSA-N
유전자 정보
human ... TH(7054)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Iodotyrosine coupled with di-iodotyrosine results in the synthesis of 3,5,3′-tri-iodothyronine (T3) or 3,3′,5′-tri-iodothyronine (rT3).
애플리케이션
3-Iodo-L-tyrosine has been used as an inhibitor for tyrosine hydroxylase enzyme in Drosophila and silkworm pupae.
생화학적/생리학적 작용
3-iodotyrosine (3-IY) inhibits tyrosine hydroxylase that catalyzes levodopa (L-DOPA) formation from tyrosine. Iodotyrosine deiodinase enzyme deficiency leads to elevated levels of 3-IY in serum and urine in severe hypothyroidism and goiter.
TH (tyrosine 3-hydroxylase) is responsible for catalyzing the first step of the noradrenergic biosynthesis pathway. Mutations in TH are associated with tyrosine hydroxylase deficiency, leading to conditions such as infantile parkinsonism and DOPA (dopamine)-responsive dystonia as well as encephalopathy with perinatal onset.
Tyrosine hydroxylase inhibitor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Jacqueline Studer et al.
Journal of forensic sciences, 59(6), 1650-1653 (2014-07-01)
Catecholamines, especially noradrenalin, are essential in the control of respiration and arousal. Thus, an impaired production of these neurotransmitters may contribute to the occurrence of sudden infant death syndrome (SIDS). The first step of the noradrenergic synthesis pathway is catalyzed
L G Harsing et al.
Neuroscience, 77(2), 419-429 (1997-03-01)
Striatal slices from the rat were preincubated with [3H]GABA and superfused in the presence of nipecotic acid and aminooxyacetic acid, inhibitors of high-affinity GABA transport and GABA aminotransferase, respectively. GABA efflux was estimated by monitoring tritium efflux, 98% of which
P F Fitzpatrick
Biochemistry, 30(15), 3658-3662 (1991-04-16)
The steady-state kinetic mechanism for rat tyrosine hydroxylase has been determined by using recombinant enzyme expressed in insect tissue culture cells. Variation of any two of the three substrates, tyrosine, 6-methyltetrahydropterin, and oxygen, together at nonsaturating concentrations of the third
Differential regulation of tyrosine hydroxylase in cuticular melanization and innate immunity in the silkworm Bombyx mori
Lee KS, et al.
Journal of Asia-Pacific Entomology, 18(4), 765-770 (2015)
Anionic iodotyrosine residues are required for iodothyronine synthesis
De Vijlder JJ and den Hartog MT
European Journal of Endocrinology, 138(2), 227-231 (1998)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.