추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
water: 10 mg/mL, clear
주관자
GlaxoSmithKline
저장 온도
room temp
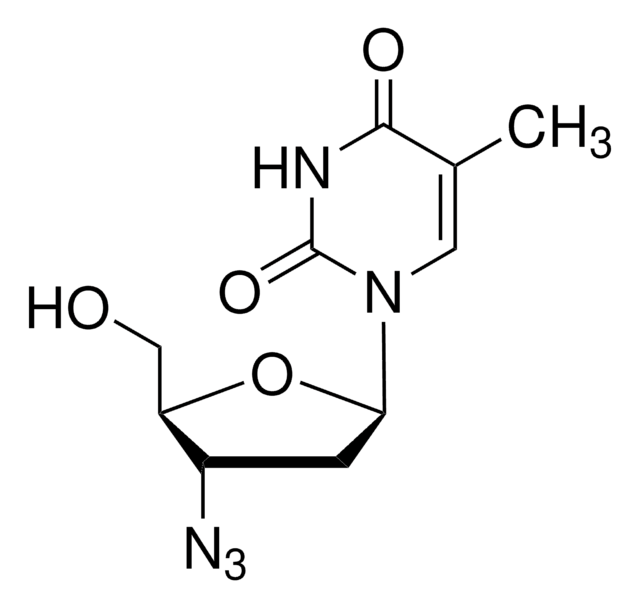
SMILES string
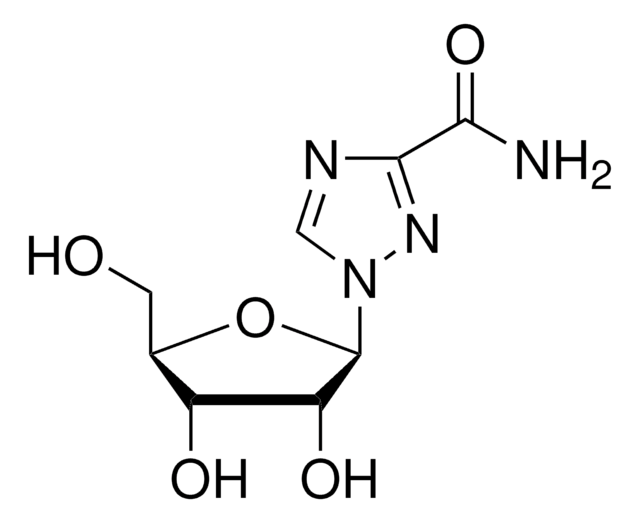
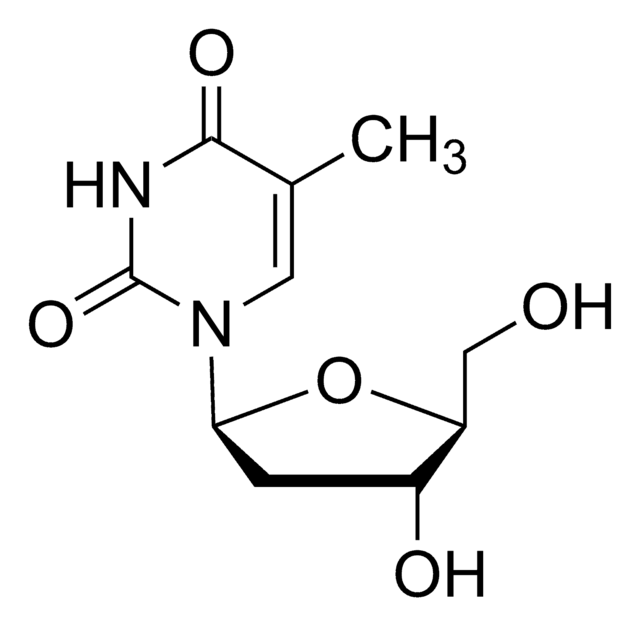
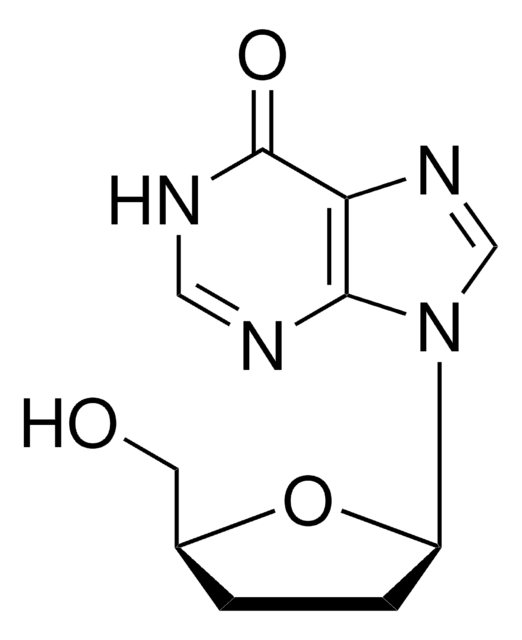
NC1=NC(=O)N(C=C1)[C@@H]2CS[C@H](CO)O2
InChI
1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m0/s1
InChI key
JTEGQNOMFQHVDC-NKWVEPMBSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Lamivudine has been used to deplete the Hepatitis B Virus (HBV) covalently closed circular DNA (cccDNA) forms for the preparation of inverse nested PCR.
생화학적/생리학적 작용
Lamivudine is a potent nucleoside analog reverse transcriptase inhibitor (nRTI). It is an analogue of cytidine, and can inhibit both types (1 and 2) of HIV reverse transcriptase as well as the reverse transcriptase of hepatitis B. It needs to be phosphorylated to its triphosphate form before it is active. 3TC-triphosphate also inhibits cellular DNA polymerase.
특징 및 장점
This compound is a featured product for ADME Tox research. Click here to discover more featured ADME Tox products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound was developed by GlaxoSmithKline. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Chia-Yen Dai et al.
The Journal of antimicrobial chemotherapy, 68(10), 2332-2338 (2013-06-27)
For hepatitis B e antigen (HBeAg)-positive patients, continuing therapy (consolidation) for 6-12 months before cessation of nucleos(t)ide analogues (NAs) was recommended. This study aimed to investigate whether a longer period of lamivudine consolidation therapy leads to better outcomes and the
Raph L Hamers et al.
Journal of acquired immune deficiency syndromes (1999), 64(2), 174-182 (2013-07-31)
This study assessed HIV-hepatitis B virus (HBV) coinfection in southern Africa in terms of prevalence, viral characteristics, occult HBV, and the effect of lamivudine- versus tenofovir-containing first-line combination antiretroviral treatment (cART) on HBV-related outcomes. A multicenter prospective cohort of HIV-infected
Sharon L Walmsley et al.
The New England journal of medicine, 369(19), 1807-1818 (2013-11-08)
Dolutegravir (S/GSK1349572), a once-daily, unboosted integrase inhibitor, was recently approved in the United States for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in combination with other antiretroviral agents. Dolutegravir, in combination with abacavir-lamivudine, may provide a simplified
Hepatitis B virus DNA integration occurs early in the viral life cycle in an in vitro infection model via NTCP-dependent uptake of enveloped virus particles
Tu T, et al.
Journal of virology, JVI-02007 (2018)
François Raffi et al.
The Lancet. Infectious diseases, 13(11), 927-935 (2013-10-01)
In the primary analysis of SPRING-2 at week 48, dolutegravir showed non-inferior efficacy to and similar tolerability to raltegravir in adults infected with HIV-1 and naive for antiretroviral treatment. We present the 96 week results. SPRING-2 is an ongoing phase
문서
당사는 다양한 항생제, 항바이러스제, 항진균제와 더불어 면역체계 신호전달 표적 식별 및 검증을 위한 작용제, 길항제, 조절제 및 기타 생체 활성 저분자를 제공합니다.
Bioactive small molecules for immune system signaling target identification/validation and antibiotics, antivirals, and antifungals offered.
Discover Bioactive Small Molecules for ADME/Tox
관련 콘텐츠
ADME/Tox를 위한 생체 활성 저분자
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.