PHR1292
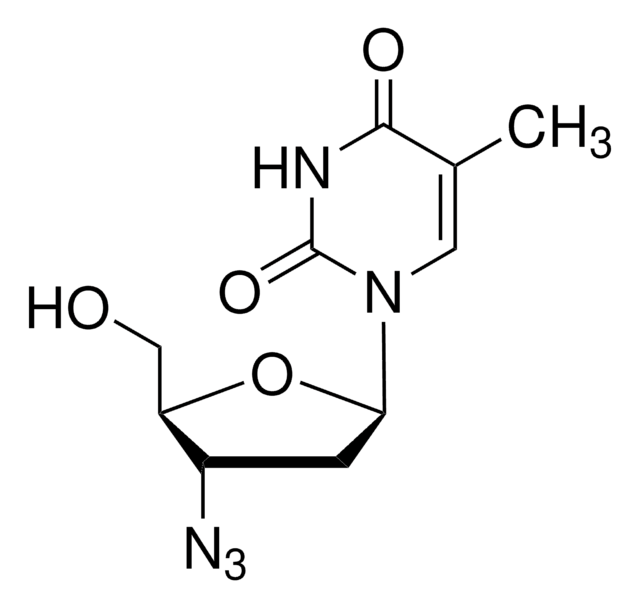
Zidovudine
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
3′-Azido-3′-deoxythymidine, AZT, Azidothymidine, ZDV, Zidovudine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C10H13N5O4
CAS Number:
Molecular Weight:
267.24
Beilstein:
3595791
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to BP 803
traceable to Ph. Eur. Z1900000
traceable to USP 1724500
API family
zidovudine
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
113-115 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CC1=CN([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)C(=O)NC1=O
InChI
1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1
InChI key
HBOMLICNUCNMMY-XLPZGREQSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Zidovudine (AZT) is a thymidine analog anti-HIV drug, known to decrease the mortality rate in patients affected with HIV. The mode of action involves inhibiting the activity of viral reverse transcriptase and interrupting HIV replication.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to in-house working standards.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to in-house working standards.
애플리케이션
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Zidovudine may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations, tablets and drug dosage forms by chromatography and spectrophotometric techniques.
생화학적/생리학적 작용
Reverse transcriptase inhibitor active against HIV-1 virus.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAA0303 in the slot below. This is an example certificate only and may not be the lot that you receive.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Muta. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Spectrophotometric determination of Zidovudine in pharmaceuticals based on charge-transfer complexation involving N-bromosuccinimide, metol and sulphanilic acid as reagents
Basavaiah K, et al.
Journal of Chemistry, 4(2), 173-179 (2007)
Alteration of zidovudine pharmacokinetics by probenecid in patients with AIDS or AIDS-related complex
de Miranda P, et al.
Clinical Pharmacology and Therapeutics, 46(5), 494-499 (1989)
Reverse Phase High Performance Liquid Chromatographic Determination Of Zidovudine And Lamivudine In Tablet Dosage Form.
Palled MS, et al.
Indian Journal of Pharmaceutical Sciences, 67(1), 110-110 (2005)
Photoelectron spectra of important drug molecules: Zidovudine and Artemisinine
Novak I and Kovac B
The Journal of Organic Chemistry, 68(14), 5777-5779 (2003)
Determination of abacavir, lamivudine and zidovudine in pharmaceutical tablets, human serum and in drug dissolution studies by HPLC
Savaser A, et al.
Chromatographia, 65(5-6), 259-265 (2007)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.