추천 제품
Quality Level
분석
>98% (HPLC)
형태
solid
색상
white
solubility
DMSO: soluble 18 mg/mL (clear yellow solution)
H2O: insoluble
저장 온도
2-8°C
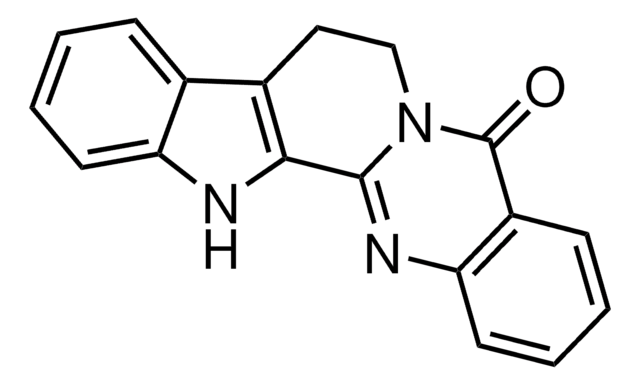
SMILES string
O=C1N2CCc3c([nH]c4ccccc34)C2=Nc5ccccc15
InChI
1S/C18H13N3O/c22-18-13-6-2-4-8-15(13)20-17-16-12(9-10-21(17)18)11-5-1-3-7-14(11)19-16/h1-8,19H,9-10H2
InChI key
ACVGWSKVRYFWRP-UHFFFAOYSA-N
유전자 정보
human ... CYP1A1(1543) , CYP1A2(1544) , CYP1B1(1545)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
Delayed rectifier K+ channel blocker. Inhibits platelet aggregation; vasoldilator.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Máté Bubenyák
Acta pharmaceutica Hungarica, 81(4), 139-149 (2012-02-15)
Quinazolinocarboline rutaecarpine and evodiamine (Evodia rutaecarpa) are main alkaloid components of traditional Chinese folk-remedies. Evodiamine exhibited selective antitumor and antimetastatic effects on several cancer cell lines and became lead structure of anticancer agents. During our synthetic research we achieved to
Chuan-Qin Hu et al.
Journal of Asian natural products research, 14(7), 634-639 (2012-05-16)
A new natural product, 10-hydroxyrutaecarpine (1), and a rarely new glycosidic alkaloid, rutaecarpine-10-O-rutinoside (2), along with the known compounds rutaecarpine (3), evodiamine, wuzhuyuamide-I, and dehydroevodiamine were isolated from the butanol fraction of 70% ethanol aqueous extract of the dried and
Seung Ill Kim et al.
Archives of pharmacal research, 35(5), 785-789 (2012-05-31)
A series of rutaecarpine derivatives were prepared by employing previously reported methods and their inhibitory activities against topoisomerase I and II were evaluated. Among them, strongly cytotoxic 10-bromorutaecarpine and 3-chlororutaecarpine showed strong inhibitory activities against topo I and II.
S N Wu et al.
Neuropharmacology, 41(7), 834-843 (2001-10-31)
The effects of rutaecarpine on ionic currents of NG108-15 neuronal cells were investigated in this study. Rutaecarpine (2-100 microM) suppressed the amplitude of delayed rectifier K+ current (I(K(DR))) in a concentration-dependent manner. The IC50 value for rutaecarpine-induced inhibition of I(K(DR))
Wenguang Yin et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 34(22), 2946-2949 (2010-03-10)
To establish a SPE-HPLC method for the determination and pharmacokinetic study of evodiamine and rutacarpine in rat plasma. A Kromasil C18 column (4.6 mm x 250 mm, 5 microm) was used with acetonitrile-water-tetrahydrofuran-acetic acid (51:48:1:0.1) as a mobile phase and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.