모든 사진(3)
About This Item
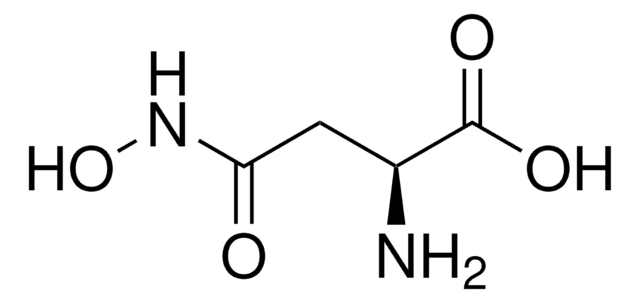
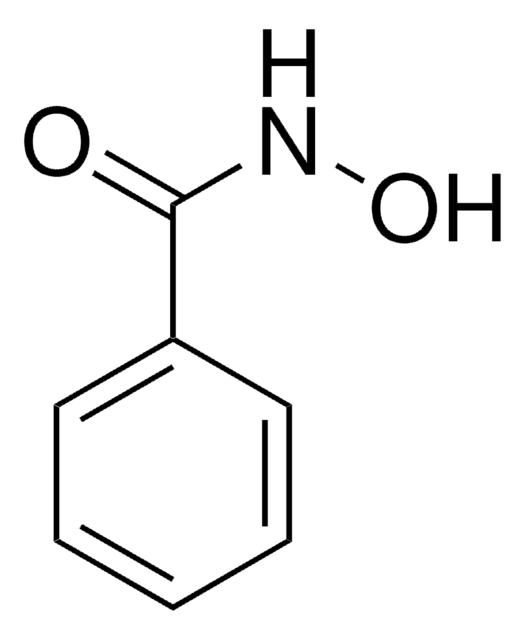
실험식(Hill 표기법):
C3H8N2O3
CAS Number:
Molecular Weight:
120.11
MDL number:
UNSPSC 코드:
12352209
PubChem Substance ID:
NACRES:
NA.26
추천 제품
제품명
DL-Serine hydroxamate, seryl-tRNA synthetase inhibitor
Quality Level
분석
≥97% (TLC)
양식
powder
기술
ligand binding assay: suitable
색상
white to off-white
응용 분야
cell analysis
저장 온도
−20°C
SMILES string
NC(CO)C(=O)NO
InChI
1S/C3H8N2O3/c4-2(1-6)3(7)5-8/h2,6,8H,1,4H2,(H,5,7)
InChI key
LELJBJGDDGUFRP-UHFFFAOYSA-N
애플리케이션
Serine has been used as an inhibitor of seryl-tRNA synthetase. DL-Serine hydroxamate is used to induce metabolic synthesis of guanosine 3′-diphosphate 5′-diphosphate (ppGpp) in E. coli by amino acid starvation. It is also used to synchronize cell cycle in E. coli cultures by inhibition of tRNA charging.
생화학적/생리학적 작용
Serine is involved in the one-carbon unit metabolism. It is associated with the biosynthesis of cysteine, ceramide, phosphatidylserine, purine and pyrimidine. In bacteria, it participates in tryptophan synthesis. Gluconeogenesis, one of the important biochemical processes, involves serine, particularly in ruminants. Protein phosphorylation is one such event that utilizes serine. Glycine, a metabolic product of serine, serves as an antioxidant and a neurotransmitter. D-serine is known to activate the N-methyl-D-aspartate (NMDA) receptors of the brain. Serine hydroxamate, a structural analogue of serine prevents seryl-tRNA (transfer ribonucleic acid) charging and thereby decreases phospholipid and nucleic acid synthesis in Escherichia coli.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Yu Zeng et al.
Antimicrobial agents and chemotherapy, 53(11), 4619-4627 (2009-09-02)
The Trojan horse antibiotic albomycin, produced by Streptomyces sp. strain ATCC 700974, contains a thioribosyl nucleoside moiety linked to a hydroxamate siderophore through a serine residue. The seryl nucleoside structure (SB-217452) is a potent inhibitor of seryl-tRNA synthetase (SerRS) in
G P van Wezel et al.
Microbiology (Reading, England), 141 ( Pt 10), 2519-2528 (1995-10-01)
In Streptomyces coelicolor A3(2), two genes, tuf1 and tuf3, encode the apparent polypeptide chain elongation factors EF-Tu1 and EF-Tu3, respectively. While tuf1 appears to code for the major EF-Tu, the function of tuf3 is unknown. To assess the role of
Yuki Matsumoto et al.
BMC genomics, 14, 808-808 (2013-11-21)
Cell growth rate reflects an organism's physiological state and largely relies on the ability of gene expression to respond to the environment. The relationship between cellular growth rate and gene expression remains unknown. Growth rate-coordinated changes in gene expression were
B Belitsky et al.
The Journal of biological chemistry, 257(9), 4677-4679 (1982-05-10)
Lack of three different amino acids or treatment with the analogue DL-serine hydroxamate does not induce the accumulation of ppGpp and pppGpp, the 3'-pyrophosphates of GDP and GTP, respectively, in Rhizobium meliloti strain 41. Surprisingly, RNA accumulation is controlled under
Effect of serine hydroxamate on phospholipid synthesis in Escherichia coli.
Pizer Ll and Merlie JP
Journal of Bacteriology, 114(3) (1973)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.