추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to light brown
solubility
DMSO: 10 meq/mL, clear
저장 온도
2-8°C
SMILES string
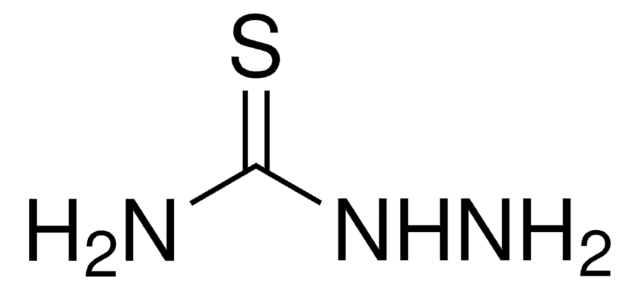
S=C(N\N=C\c1ncccc1N)N
InChI
1S/C7H9N5S/c8-5-2-1-3-10-6(5)4-11-12-7(9)13/h1-4H,8H2,(H3,9,12,13)/b11-4+
InChI key
XMYKNCNAZKMVQN-NYYWCZLTSA-N
생화학적/생리학적 작용
3-Aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) is a ribonucleotide reductase inhibitor and iron chelator with anti-tumor activity.
3-Aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) is a ribonucleotide reductase inhibitor and iron chelator with anti-tumor activity.
3-aminopyridine carboxaldehyde thiosemicarbazone (3-AP) has a IC50 value of 0.3μM. It exhibits anti-proliferative activity in preclinical models of cancer, such as lung cancer. It also has an ability to increase the cytotoxicity, intracellular uptake and DNA incorporation of gemcitabine in vitro.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine?) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells.
Ma B, et al.
Investigational New Drugs, 26(2), 169-173 (2008)
Jack C Yalowich et al.
Biochemical pharmacology, 84(1), 52-58 (2012-04-17)
The thiosemicarbazones Dp44mT (di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone) and triapine have potent antiproliferative activity and have been evaluated as anticancer agents. While these compounds strongly bind iron and copper, their mechanism(s) of action are incompletely understood. A recent report (Rao et al., Cancer Research
Ana Popović-Bijelić et al.
Journal of inorganic biochemistry, 105(11), 1422-1431 (2011-10-01)
Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone, 3-AP) is currently the most promising chemotherapeutic compound among the class of α-N-heterocyclic thiosemicarbazones. Here we report further insights into the mechanism(s) of anticancer drug activity and inhibition of mouse ribonucleotide reductase (RNR) by Triapine. In addition
Charles A Kunos et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 16(4), 1298-1306 (2010-02-11)
This study assessed the safety/tolerability, pharmacokinetics, and clinical activity of three times weekly i.v. 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in combination with once-weekly i.v. cisplatin and daily pelvic radiation in patients with gynecologic malignancies. 3-AP is a novel small-molecule inhibitor
Charles A Kunos et al.
Radiation research, 174(5), 574-581 (2010-10-20)
For repair of damaged DNA, cells increase de novo synthesis of deoxyribonucleotide triphosphates through the rate-limiting, p53-regulated ribonucleotide reductase (RNR) enzyme. In this study we investigated whether pharmacological inhibition of RNR by 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) enhanced chemoradiation sensitivity
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.