1009923
USP
Acetyltriethyl citrate
United States Pharmacopeia (USP) Reference Standard
동의어(들):
Triethyl 2-acetylcitrate, Acetyl triethyl citrate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
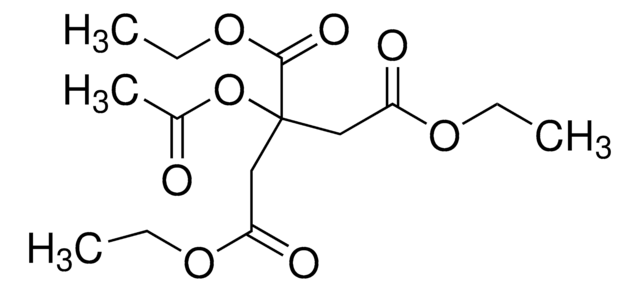
CH3COOC(COOCH2CH3)(CH2COOCH2CH3)2
CAS Number:
Molecular Weight:
318.32
Beilstein:
1804947
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
triethyl citrate
제조업체/상표
USP
refractive index
n20/D 1.439 (lit.)
bp
228-229 °C/100 mmHg (lit.)
density
1.136 g/mL at 25 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
O=C(OCC)C(CC(OCC)=O)(CC(OCC)=O)OC(C)=O
InChI
1S/C14H22O8/c1-5-19-11(16)8-14(22-10(4)15,13(18)21-7-3)9-12(17)20-6-2/h5-9H2,1-4H3
InChI key
WEAPVABOECTMGR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Acetyltriethyl citrate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Friederike Eisenächer et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 88(3), 778-786 (2014-08-03)
Gastroretentive drug delivery systems are retained in the stomach for a sufficient time interval, releasing the drug in a controlled manner. According to literature, the floating principle is the most frequently used formulation approach for gastric retention. However, many publications
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.