1279000
USP
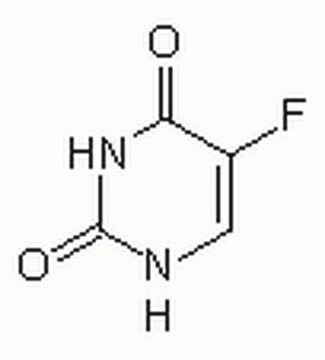
Fluorouracil
United States Pharmacopeia (USP) Reference Standard
동의어(들):
5-Fluorouracil, 2,4-Dihydroxy-5-fluoropyrimidine, 5-FU, 5-Fluoro-2,4(1H,3H)-pyrimidinedione
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C4H3FN2O2
CAS Number:
Molecular Weight:
130.08
Beilstein:
127172
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
fluorouracil
제조업체/상표
USP
mp
282-286 °C (dec.) (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
FC1=CNC(=O)NC1=O
InChI
1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
InChI key
GHASVSINZRGABV-UHFFFAOYSA-N
유전자 정보
human ... TYMS(7298)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Fluorouracil USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Flucytosine
- Flucytosine Capsules
- Fluorouracil
- Fluorouracil Cream
- Fluorouracil Injection
- Fluorouracil Topical Solution
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Carc. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
S López-Estévez et al.
Gene therapy, 21(7), 673-681 (2014-05-09)
Suicide gene therapy (SGT) is a promising strategy for treating cancer. In this work, we show that thymidine phosphorylase (TP) deficiency, the underlying genetic defect in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), presents an opportunity to apply SGT using capecitabine, a commonly
Tania Diaz et al.
Journal of surgical oncology, 109(7), 676-683 (2014-02-11)
Surgery is the standard treatment for colorectal cancer (CRC), and adjuvant chemotherapy has been shown to be effective in stage III but less so in stage II. We have analyzed the expression of the miR-200 family in tissue samples from
John M L Ebos et al.
EMBO molecular medicine, 6(12), 1561-1576 (2014-11-02)
Thousands of cancer patients are currently in clinical trials evaluating antiangiogenic therapy in the neoadjuvant setting, which is the treatment of localized primary tumors prior to surgical intervention. The rationale is that shrinking a tumor will improve surgical outcomes and
Zhe Guo et al.
Annals of surgical oncology, 21(9), 3069-3076 (2014-04-15)
It is unclear whether hepatic resection (HR) or transarterial chemoembolization (TACE) is associated with better outcomes for patients with hepatocellular carcinoma (HCC) in Barcelona Clinic Liver Cancer (BCLC) stage A. The present study compared survival for patients with BCLC stage
Comparative economics of a 12-gene assay for predicting risk of recurrence in stage II colon cancer.
Steven R Alberts et al.
PharmacoEconomics, 32(12), 1231-1243 (2014-08-27)
Prior economic analysis that compared the 12-gene assay to published patterns of care predicted the assay would improve outcomes while lowering medical costs for stage II, T3, mismatch-repair-proficient (MMR-P) colon cancer patients. This study assessed the validity of those findings
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.