추천 제품
Grade
pharmaceutical primary standard
API family
indapamide
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
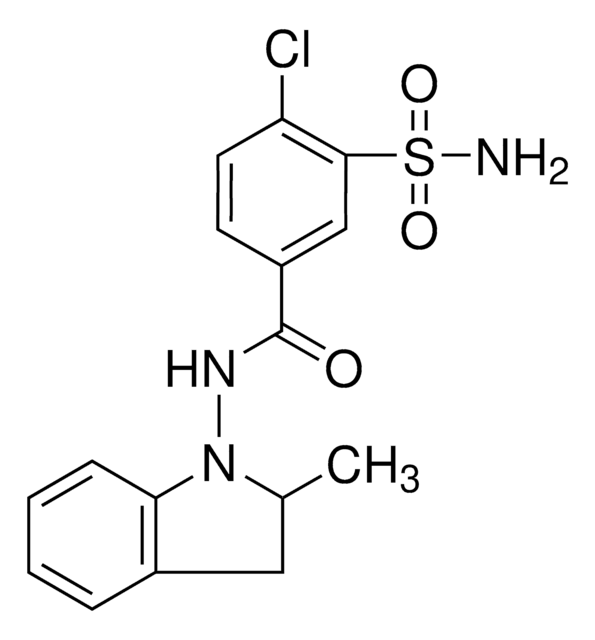
SMILES string
CC1Cc2ccccc2N1NC(=O)c3ccc(Cl)c(c3)S(N)(=O)=O
InChI
1S/C16H16ClN3O3S/c1-10-8-11-4-2-3-5-14(11)20(10)19-16(21)12-6-7-13(17)15(9-12)24(18,22)23/h2-7,9-10H,8H2,1H3,(H,19,21)(H2,18,22,23)
InChI key
NDDAHWYSQHTHNT-UHFFFAOYSA-N
유전자 정보
human ... SLC12A3(6559)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Indapamide USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Indapamide Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Lact. - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
J Sassard et al.
Fundamental & clinical pharmacology, 19(6), 637-645 (2005-11-30)
The relationship between blood pressure (BP) and cardiovascular risk is clearly established; hypertension increases the rate of cardiovascular. High systolic blood pressure (SBP) may be the main parameter involved in cardiovascular morbidity and mortality. The benefit of lowering BP, particularly
Stéphane Laurent
Journal of hypertension. Supplement : official journal of the International Society of Hypertension, 21(3), S11-S18 (2003-08-22)
To review the safety and efficacy of the very-low-dose combination of perindopril 2 mg and indapamide 0.625 mg (Per2/Ind0.625) in essential hypertension, in relation to blood pressure control and target-organ damage. We included in this review several double-blind, randomized studies
John Chalmers et al.
Journal of hypertension. Supplement : official journal of the International Society of Hypertension, 26(3), S21-S27 (2009-04-15)
The ADVANCE trial was designed to determine the effects of routine blood pressure lowering using a fixed combination of perindopril-indapamide on major vascular outcomes in patients with type 2 diabetes, regardless of initial blood pressure levels or the use of
A J Matheson et al.
Drugs, 61(8), 1211-1229 (2001-07-24)
The fixed low-dose combination of the ACE inhibitor perindopril and the non-thiazide diuretic indapamide has been evaluated in the management of patients with mild to moderate hypertension. Combination therapy aims to improve overall therapeutic efficacy while minimising adverse effects. In
J J Mourad et al.
Current medical research and opinion, 25(9), 2271-2280 (2009-07-25)
Despite the widespread notion that controlling hypertension is essential to improve cardiovascular outcome, uncontrolled hypertension rates remain high. Fixed-dose combinations are used routinely to reduce the impact of hypertension. Treatment with fixed-combination perindopril/indapamide, for example, at the currently approved doses
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.