107360
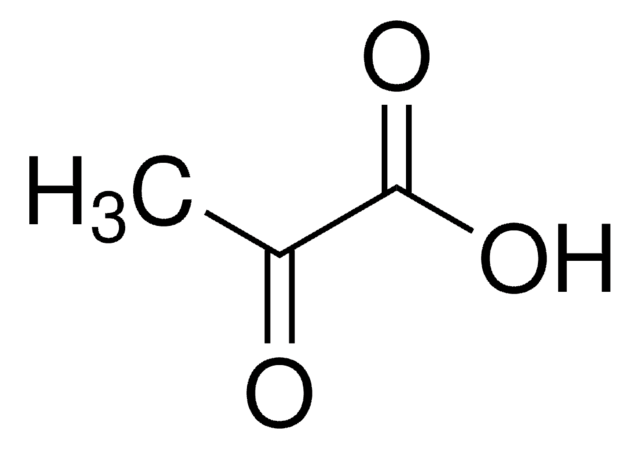
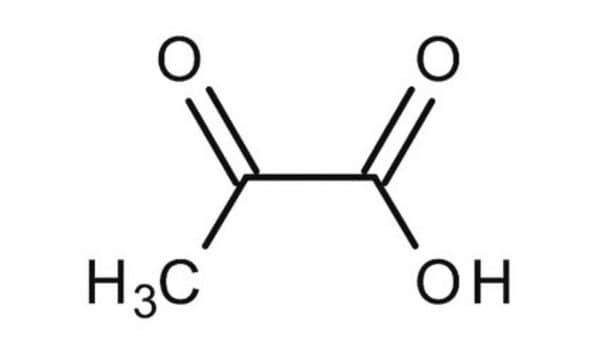
Pyruvic acid
98%
Synonym(s):
α-Ketopropionic acid, 2-Oxopropionic acid
About This Item
Recommended Products
assay
98%
refractive index
n20/D 1.428 (lit.)
bp
165 °C (lit.)
mp
11-12 °C (lit.)
solubility
H2O: miscible
alcohol: miscible
diethyl ether: miscible
density
1.267 g/mL at 25 °C (lit.)
functional group
carboxylic acid
ketone
storage temp.
2-8°C
SMILES string
CC(=O)C(O)=O
InChI
1S/C3H4O3/c1-2(4)3(5)6/h1H3,(H,5,6)
InChI key
LCTONWCANYUPML-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1C
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
183.2 °F - closed cup
flash_point_c
84 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Science Slam panel: Leading gene therapy developers discuss commercialization challenges and the importance of robust process development plans.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service