123838
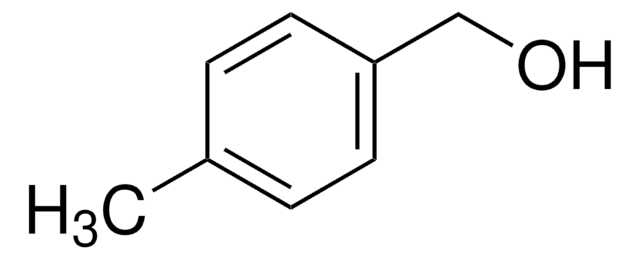
Biphenyl-4-methanol
98%
Synonym(s):
4-(Hydroxymethyl)biphenyl, 4-Phenylbenzyl alcohol
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
C6H5C6H4CH2OH
CAS Number:
Molecular Weight:
184.23
Beilstein/REAXYS Number:
1937761
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
96-100 °C (lit.)
solubility
acetone: soluble 25 mg/mL
functional group
hydroxyl
phenyl
SMILES string
OCc1ccc(cc1)-c2ccccc2
InChI
1S/C13H12O/c14-10-11-6-8-13(9-7-11)12-4-2-1-3-5-12/h1-9,14H,10H2
InChI key
AXCHZLOJGKSWLV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Biphenyl-4-methanol acts as monofunctional alcohol initiator during the ring-opening polymerization of trimethylene carbonate (TMC) catalyzed by CH3SO3H and copolymerisation of ε-caprolactone and TMC.
Application
Biphenyl-4-methanol was used as reagent for the rapid quantitative determination of lithium alkyls.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Journal of Organic Chemistry, 48, 2603-2603 (1983)
Induction of morphological transformation in C3H/10T1/2 clone 8 cells.
A Poole et al.
Mutation research, 100(1-4), 219-221 (1982-01-01)
Copolymerisation of e-caprolactone and trimethylene carbonate catalysed by methanesulfonic acid.
Campos JM, et al.
Eur. Polymer J., 49(12), 4025-4034 (2013)
The cytotoxic and mutagenic effects of 4CMB, BC and 4HMB in V79 Chinese hamster cells.
F Mirzayans et al.
Mutation research, 100(1-4), 239-244 (1982-01-01)
Ryo Mizoguchi et al.
Molecular pharmaceutics, 16(5), 2142-2152 (2019-04-05)
Co-amorphous technology was recently introduced to stabilize drugs in the amorphous state for drug development. We examined the predictability of the formation of co-amorphous systems and identified two reliable indicators of successful formation: (1) a negative Δ Hmix value and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service