140023
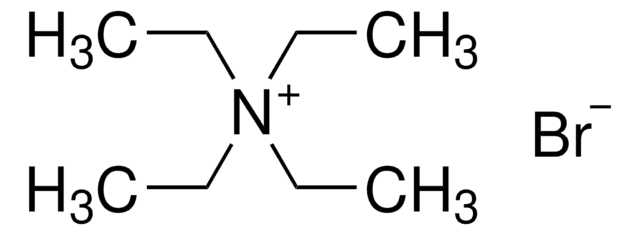
Tetraethylammonium bromide
reagent grade, 98%
Synonym(s):
TEAB, TEA bromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C2H5)4N(Br)
CAS Number:
Molecular Weight:
210.16
Beilstein/REAXYS Number:
3563430
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
reagent grade
Quality Level
assay
98%
form
crystalline
impurities
≤2% triethylamine hydrobromide
pH
6.5 (100 g/L)
mp
285 °C (dec.) (lit.)
SMILES string
[Br-].CC[N+](CC)(CC)CC
InChI
1S/C8H20N.BrH/c1-5-9(6-2,7-3)8-4;/h5-8H2,1-4H3;1H/q+1;/p-1
InChI key
HWCKGOZZJDHMNC-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Tetraethylammonium bromide may be used:
- As an organic template for synthesizing zeolite beta via hydrothermal crystallization.
- As a catalyst for the oxidative coupling of aldehydes or alcohols with thiophenols or disulfides to form thioesters.
- In combination with o-iodoxybenzoic acid (IBX) for the oxidative dehomologation of primary carboxamides to nitriles and α,α-disubstituted acetamides to ketones. This reagent combination can also be used for the conversion of sulfides to sulfoxides and N,N-disubstituted glycylamides into corresponding cyanamides.
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 3
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
361.4 °F - closed cup
flash_point_c
183 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Novel and facile transformation of N, N-disubstituted glycylamides into corresponding cyanamides by using pentavalent iodine reagents in combination with tetraethylammonium bromide.
Chaudhari K
Synlett, 18, 2815-2818 (2007)
Oxidative Conversion of ?, ?-Disubstituted Acetamides to Corresponding One-Carbon-Shorter Ketones Using Hypervalent Iodine (?5) Reagents in Combination with Tetraethylammonium Bromide.
Bellale E
The Journal of Organic Chemistry, 73(23), 9473-9475 (2008)
A mild, chemoselective oxidation of sulfides to sulfoxides using o-iodoxybenzoic acid and tetraethylammonium bromide as catalyst.
Shukla V
The Journal of Organic Chemistry, 68(13), 5422-5425 (2003)
Hydrothermal crystallization of zeolite beta using tetraethylammonium bromide.
Eapen M
Zeolites, 14(4), 295-302 (1994)
Tetraethylammonium Bromide?Catalyzed Oxidative Thioesterification of Aldehydes and Alcohols.
Zhu X
Advanced Synthesis & Catalysis, 355(18), 3558-3562 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service