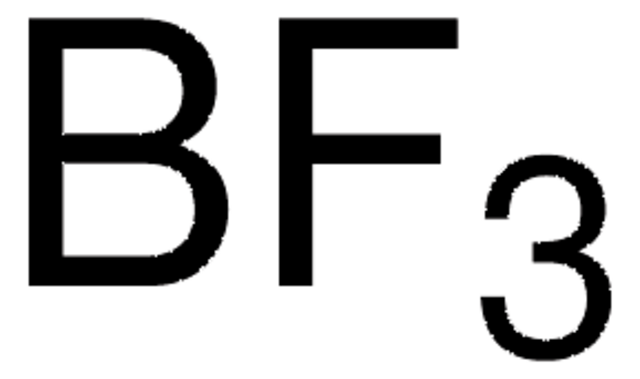261998
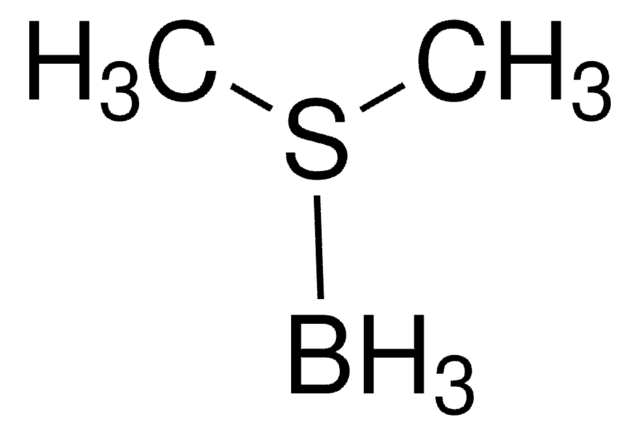
Boron trifluoride methyl sulfide complex
95%
Synonym(s):
Dimethyl sulfide-trifluoroborane, Trifluoroborane-methyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2S · BF3
CAS Number:
Molecular Weight:
129.94
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
liquid
reaction suitability
core: boron
reagent type: Lewis acid
reagent type: catalyst
density
1.235 g/mL at 25 °C (lit.)
functional group
thioether
SMILES string
C[S+](C)[B-](F)(F)F
InChI
1S/C2H6BF3S/c1-7(2)3(4,5)6/h1-2H3
InChI key
VPECYQXBNOAJIF-UHFFFAOYSA-N
Application
Boron trifluoride methyl sulfide complex is a common reagent used for dealkylation of ethers. It can also be used to synthesize boron trifluoride complex with phosphine ligands.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
3.2 °F - closed cup
flash_point_c
-16 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structural optimization of 2, 5-thiophene amides as highly potent and selective 17?-hydroxysteroid dehydrogenase type 2 inhibitors for the treatment of osteoporosis.
Marchais-Oberwinkler S, et al.
Journal of Medicinal Chemistry, 56(1), 167-181 (2012)
Systematics of BX3 and BX2+ Complexes (X= F, Cl, Br, I) with Neutral Diphosphine and Diarsine Ligands.
Burt J, et al.
Inorganic Chemistry, 55(17), 8852-8864 (2016)
Experimental and computational exploration of the dynamic behavior of (PNP) BF 2, a boron compound supported by an amido/bis (phosphine) pincer ligand.
DeMott JC
Dalton Transactions, 40(43), 11562-11570 (2011)
Understanding the Reactivity of Enol Ether Radical Cations: Investigation of Anodic Four-Membered Carbon Ring Formation.
Yamaguchi Y, et al.
The Journal of Organic Chemistry, 78(6), 2626-2638 (2013)
Isoxazole substituted chromans against oxidative stress-induced neuronal damage.
Koufaki M
Bioorganic & Medicinal Chemistry, 19(16), 4841-4850 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service