287512
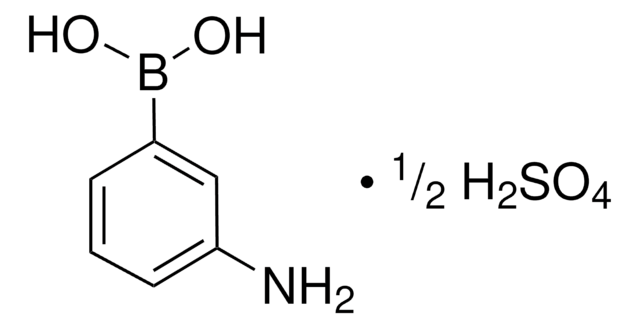
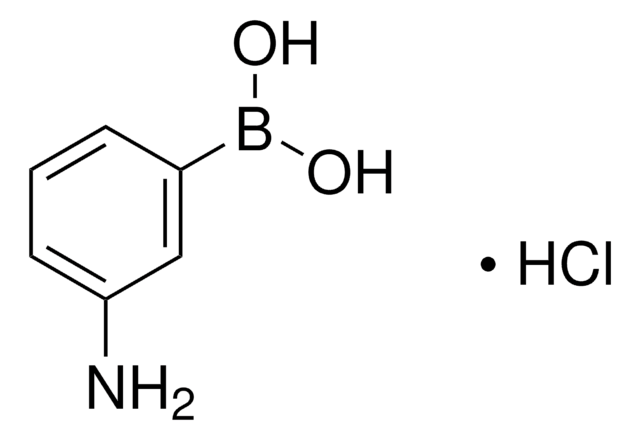
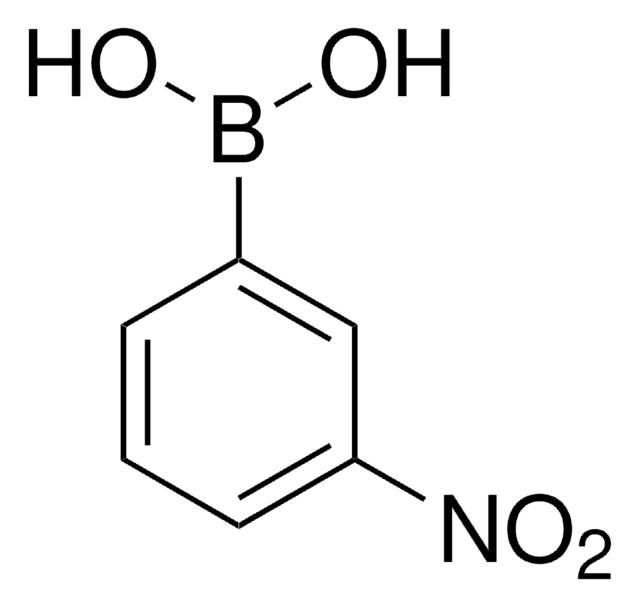
3-Aminophenylboronic acid monohydrate
98%
Synonym(s):
3-Aminobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H4B(OH)2 · H2O
CAS Number:
Molecular Weight:
154.96
Beilstein/REAXYS Number:
2936342
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
93-96 °C (lit.)
SMILES string
[H]O[H].Nc1cccc(c1)B(O)O
InChI
1S/C6H8BNO2.H2O/c8-6-3-1-2-5(4-6)7(9)10;/h1-4,9-10H,8H2;1H2
InChI key
XAEOVQODHLLNKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent used for
Reagent used for Preparation of
- Suzuki-Miyaura cross-coupling
Reagent used for Preparation of
- Gram-positive antivirulence drugs and inhibitors of Streptococcus agalactiae Stk1
- Regioisomer of Zaleplon (a sedative)
- Amphiphilic random glycopolymer, which self-assemble to form nanoparticles, with potential as a glucose-sensitive matrix
- Chemomechanical polymer that expands and contracts in blood plasma with high glucose selectivity
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic studies connected with the preparation of N-[3-(3-cyanopyrazolo[1,5-a]pyrimidin-5-yl)phenyl]-N-ethylacetamide, a zaleplon regioisomer
Radl, S.; et al.
Heterocycles, 80, 1359-1379 (2010)
Qianqian Guo et al.
Journal of biomaterials science. Polymer edition, 30(10), 815-831 (2019-05-03)
We reported on the fabrication of sugar-responsive nanogels covalently incorporated with 3-acrylamidophenylboronic acid (AAPBA) as glucose-recognizing moiety, 2-(acrylamido)glucopyranose (AGA) as biocompatible moiety, and boron dipyrromethene (BODIPYMA) as fluorescence donor molecule. The p(AAPBA-AGA-BODIPYMA) nanogels were synthesized via reversible addition-fragmentation chain transfer
Jiangying Zhu et al.
Materials science & engineering. C, Materials for biological applications, 117, 111273-111273 (2020-09-14)
In this work, poly(ethylene glycol)-b-poly[3-acrylamidophenylboronic acid-co-styrene] (PEG-b-P(PBA-co-St) has been firstly synthesized for loading of insulin to form insulin-loaded micelles. Insulin-loaded micelles (ILM) and epidermal growth factor (EGF) are further embedded into the composite hydrogels that can be rapidly gelled by
A chemomechanical polymer that functions in blood plasma with high glucose selectivity.
George K Samoei et al.
Angewandte Chemie (International ed. in English), 45(32), 5319-5322 (2006-08-24)
Mayalen Oxoby et al.
Bioorganic & medicinal chemistry letters, 20(12), 3486-3490 (2010-06-10)
A structure-activity relationship study from a screening hit and structure-based design strategy has led to the identification of bisarylureas as potent inhibitors of Streptococcus agalactiae Stk1. As this target has been directly linked to bacterial virulence, these inhibitors can be
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service