341436
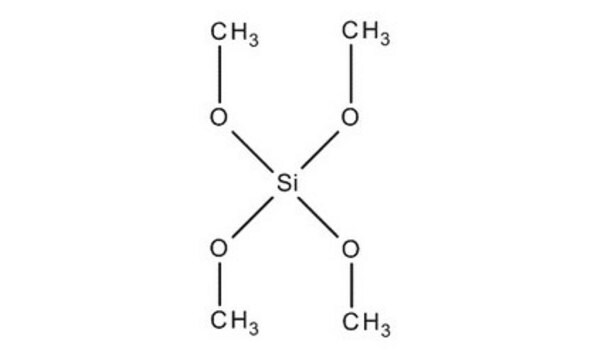
Tetramethyl orthosilicate
≥99%
Synonym(s):
Tetramethoxysilane
About This Item
Recommended Products
vapor density
5.25 (vs air)
Quality Level
vapor pressure
13 hPa ( 20 °C)
assay
≥99%
form
liquid
refractive index
n20/D 1.368 (lit.)
bp
121-122 °C (lit.)
mp
−4 °C (lit.)
density
1.023 g/mL at 25 °C (lit.)
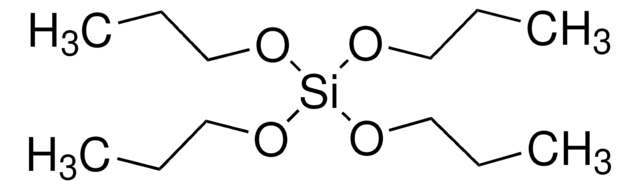
SMILES string
CO[Si](OC)(OC)OC
InChI
1S/C4H12O4Si/c1-5-9(6-2,7-3)8-4/h1-4H3
InChI key
LFQCEHFDDXELDD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a precursor to synthesize organic-inorganic coating materials by sol-gel processing method.
- As an efficient reagent for direct amidation of aliphatic and aromatic carboxylic acids with amines and anilines.
- As a precursor to fabricate SiO2 nanocomposite films by chemical vapor deposition(CVD) method.
- As a selective catalyst ofC3-methylation of indole.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
78.8 °F - closed cup
flash_point_c
26 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Reactive silicone chemistry: Focus on pure silicon production, polymerizations, and controlled stereochemistry reactions.
Silica's versatility spans various industries, including biomedical applications.
Synthesis of Melting Gels Using Mono-Substituted and Di-Substituted Alkoxysiloxanes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service