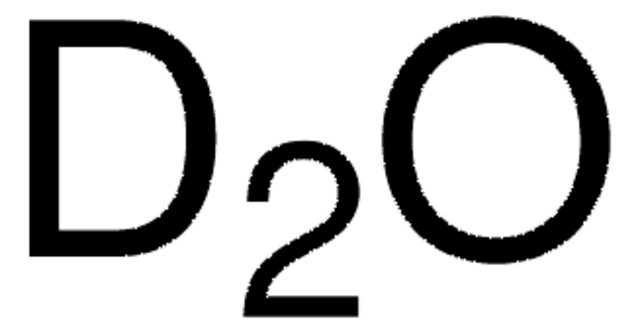343773
Deuterium oxide
99.9 atom % D, contains 1 % (w/w) 3-(trimethylsilyl)-1-propanesulfonic acid, sodium salt (DSS)
Synonym(s):
Heavy water, Water-d2
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(2)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
D2O
CAS Number:
Molecular Weight:
20.03
EC Number:
MDL number:
UNSPSC Code:
12142201
PubChem Substance ID:
NACRES:
NA.21
technique(s):
NMR: suitable
bp:
101.4 °C (lit.)
Recommended Products
isotopic purity
99.9 atom % D
Quality Level
form
liquid
contains
1 % (w/w) 3-(trimethylsilyl)-1-propanesulfonic acid, sodium salt (DSS)
technique(s)
NMR: suitable
bp
101.4 °C (lit.)
mp
3.8 °C (lit.)
SMILES string
[2H]O[2H]
InChI
1S/H2O/h1H2/i/hD2
InChI key
XLYOFNOQVPJJNP-ZSJDYOACSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Deuterium oxide can serve as a solvent in the :
- NMR analysis.
- 1HDOSY (Diffusion-Ordered Spectroscopy) NMR experiments.
Recommended products
Check out ChemisTwin®, our brand new online portal for identity confirmation and quantification of NMR spectra. Learn more or reach out to us for a free trial.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The ionization constant of deuterium oxide from 5 to 50?.
Covington AK, et al.
The Journal of Physical Chemistry, 70(12), 3820-3824 (1966)
Structure of water and hydrophobic bonding in proteins. IV. The thermodynamic properties of liquid deuterium oxide.
Nemethy G and Scheraga HA.
J. Chem. Phys. , 41(3), 680-689 (1964)
Using high-performance quantitative NMR (HP-qNMR?) for certifying traceable and highly accurate purity values of organic reference materials with uncertainties< 0.1%.
Weber M, et al.
Accreditation and Quality Assurance, 18(2), 91-98 (2013)
Lars Holm et al.
American journal of physiology. Endocrinology and metabolism, 304(8), E895-E907 (2013-02-21)
A method to determine the rate of protein breakdown in individual proteins was developed and tested in rats and confirmed in humans, using administration of deuterium oxide and incorporation of the deuterium into alanine that was subsequently incorporated into body
Takhar Kasumov et al.
American journal of physiology. Heart and circulatory physiology, 304(9), H1201-H1214 (2013-03-05)
Traditional proteomics provides static assessment of protein content, but not synthetic rates. Recently, proteome dynamics with heavy water ((2)H2O) was introduced, where (2)H labels amino acids that are incorporated into proteins, and the synthesis rate of individual proteins is calculated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service