All Photos(1)
About This Item
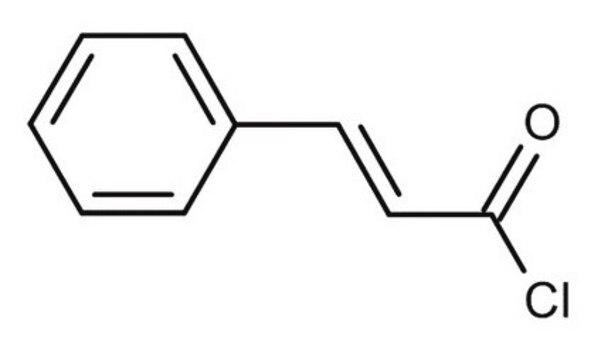
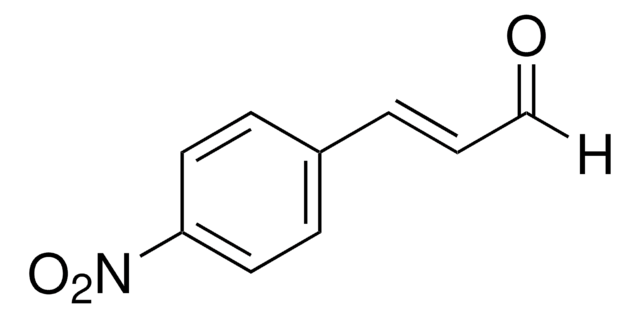
Linear Formula:
O2NC6H4CH=CHCOCl
CAS Number:
Molecular Weight:
211.60
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
powder
mp
150-153 °C (lit.)
functional group
acyl chloride
nitro
SMILES string
[O-][N+](=O)c1ccc(\C=C\C(Cl)=O)cc1
InChI
1S/C9H6ClNO3/c10-9(12)6-3-7-1-4-8(5-2-7)11(13)14/h1-6H/b6-3+
InChI key
RUPXNPWALFDXJD-ZZXKWVIFSA-N
Related Categories
Application
trans-4-Nitrocinnamoyl chloride may be used as derivatization reagent for the analysis of perhexiline and its monohydroxy metabolite in plasma by HPLC. It may be used as derivatization reagent to investigate the in vitro enzyme kinetics and CYP isoform selectivity of perhexiline monohydroxylation using human liver microsomes.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N Grgurinovich
Journal of chromatography. B, Biomedical sciences and applications, 696(1), 75-80 (1997-08-15)
A high-performance liquid chromatographic method for the analysis of perhexiline and its monohydroxy metabolite in plasma has been developed. After a simple extraction procedure, the analytes are derivatized over a 30-min period with trans-4-nitrocinnamoyl chloride. The derivatized products are monitored
L B Sørensen et al.
British journal of clinical pharmacology, 55(6), 635-638 (2003-06-20)
The aims of this study were to examine the in vitro enzyme kinetics and CYP isoform selectivity of perhexiline monohydroxylation using human liver microsomes. Conversion of rac-perhexiline to monohydroxyperhexiline by human liver microsomes was assessed using a high-performance liquid chromatography
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service