All Photos(1)
About This Item
Empirical Formula (Hill Notation):
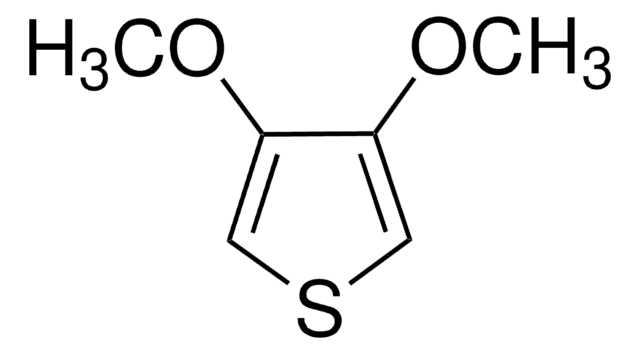
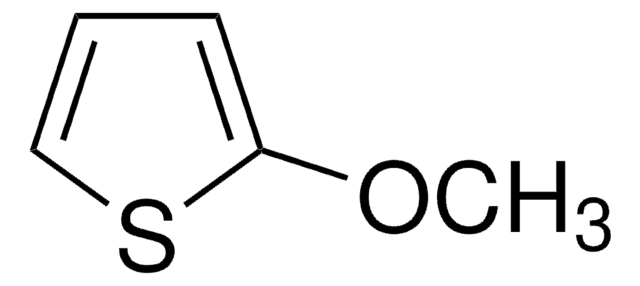
C5H6OS
CAS Number:
Molecular Weight:
114.17
Beilstein/REAXYS Number:
106404
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.532 (lit.)
bp
80-82 °C/65 mmHg (lit.)
density
1.143 g/mL at 25 °C (lit.)
SMILES string
COc1ccsc1
InChI
1S/C5H6OS/c1-6-5-2-3-7-4-5/h2-4H,1H3
InChI key
RFSKGCVUDQRZSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Methoxythiophene is a thiophene. The intramolecular and intermolecular geometries of crystals of 3-methoxythiophene were studied. Spectroscopic studies of bipolarons derived from oligomerized 3-methoxythiophene in solution has been reported. The electropolymerization of 3-methoxythiophene on Pt and Fe electrodes in an aqueous micellar medium containing sodium dodecyl sulfate and 10-3M bithiophene has been reported. Thin polymer films of 3-methoxythiophene at the cathode in a direct current discharge have been prepared.
signalword
Warning
hcodes
Hazard Classifications
Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
120.2 °F - closed cup
flash_point_c
49 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electrosynthesis and characterization of poly (3-methoxythiophene)-polybithiophene composite films prepared in micellar media on Pt and Fe substrates.
Dieng MaM, et al.
Physical Chemistry Chemical Physics, 1(8), 1731-1734 (1999)
Polymerization of 3-Methoxythiophene in a Direct-Current Discharge.
Drachev AI, et al.
High Energy Chemistry, 39(5), 333-336 (2005)
Paolo Bollella et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 21(1), 120-128 (2019-08-14)
Biocatalytic buckypaper electrodes modified with pyrroloquinoline quinone (PQQ)-dependent glucose dehydrogenase and bilirubin oxidase for glucose oxidation and oxygen reduction, respectively, were prepared for their use in a biofuel cell. A small (millimeter-scale; 2×3×2 mm3 ) enzyme-based biofuel cell was tested in
Martina Marinelli et al.
Chirality, 32(12), 1361-1376 (2020-11-17)
Novel optically active oligothiophenes bearing electron-donating chiral side chains have been prepared by synthetic methods suitable to achieve regioregular head-to-tail and head-to-head/tail-to-tail derivatives. In particular, the chiral (S)-(2-methyl)butyl moiety was linked at position 3 of the thiophene ring through heteroatoms
Spectroscopic studies of bipolarons from oligomerized 3-methoxythiophene in solution.
Chang A-C and Miller LL.
Synthetic Metals, 22(1), 71-78 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service