440736
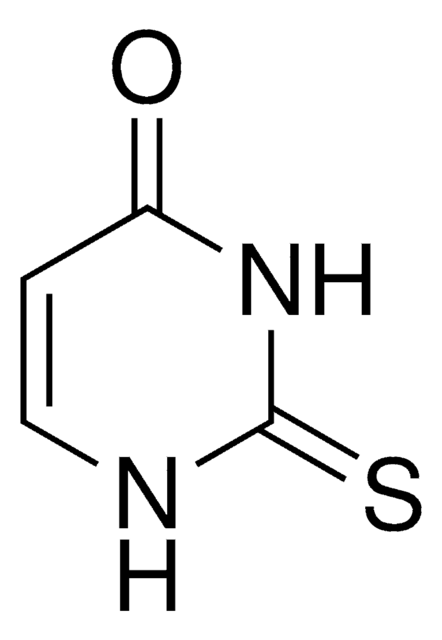
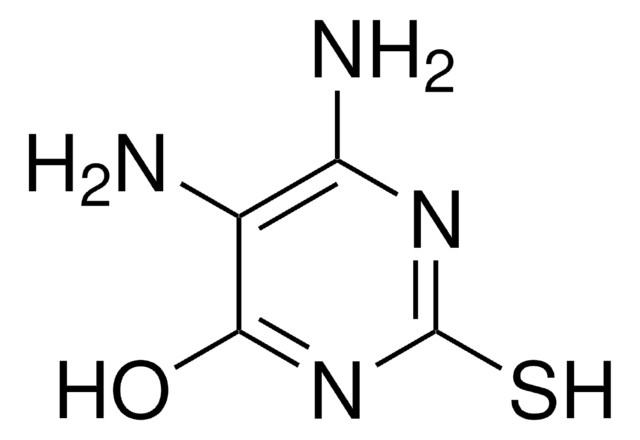
4-Thiouracil
97%
Synonym(s):
2-Hydroxy-4-mercaptopyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4N2OS
CAS Number:
Molecular Weight:
128.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
powder
mp
295 °C (dec.) (lit.)
solubility
1 M NaOH: soluble 50 mg/mL
SMILES string
Oc1nccc(S)n1
InChI
1S/C4H4N2OS/c7-4-5-2-1-3(8)6-4/h1-2H,(H2,5,6,7,8)
InChI key
OVONXEQGWXGFJD-UHFFFAOYSA-N
Related Categories
Application
4-Thiouracil is suitable reagent employed in Schneider′s media for the embryos during RNA extraction. It may be employed as reagent for the Northwestern blotting technique.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jeanine S Morey et al.
PloS one, 8(6), e66347-e66347 (2013-06-19)
Dinoflagellates possess many physiological processes that appear to be under post-transcriptional control. However, the extent to which their genes are regulated post-transcriptionally remains unresolved. To gain insight into the roles of differential mRNA stability and de novo transcription in dinoflagellates
Structure and tautomerism of the neutral and monoanionic forms of 4-thiouracil derivatives.
A Psoda et al.
Journal of the American Chemical Society, 96(22), 6832-6839 (1974-10-30)
Margaret A Nakamoto et al.
RNA (New York, N.Y.), 23(12), 1834-1849 (2017-08-31)
RNA contains over 100 modified nucleotides that are created post-transcriptionally, among which pseudouridine (Ψ) is one of the most abundant. Although it was one of the first modifications discovered, the biological role of this modification is still not fully understood.
Qing S Wang et al.
RNA (New York, N.Y.), 11(4), 404-411 (2005-02-11)
Isolating the core functional elements of an RNA is normally performed during the characterization of a new RNA in order to simplify further biochemical analysis. The removal of extraneous sequence is challenging and can lead to biases that result from
Linda Warfield et al.
Molecular cell, 68(1), 118-129 (2017-09-19)
Previous studies suggested that expression of most yeast mRNAs is dominated by either transcription factor TFIID or SAGA. We re-examined the role of TFIID by rapid depletion of S. cerevisiae TFIID subunits and measurement of changes in nascent transcription. We find that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service