662283
Bis(tert-butylcarbonyloxy)iodobenzene
97%
Synonym(s):
(Di-tert-butylcarbonyloxyiodo)benzol, 2,2-Dimethylpropanoic acid, phenyliodine complex, Bis(2,2-dimethylpropanoato-O)phenyliodide, Di-(Pivaloyloxy)iodobenzene
About This Item
Recommended Products
Quality Level
assay
97%
form
solid
reaction suitability
reagent type: catalyst
reaction type: C-H Activation
reagent type: oxidant
mp
104-109 °C
SMILES string
CC(C)(C)C(=O)O[I](OC(=O)C(C)(C)C)c1ccccc1
InChI
1S/C16H23IO4/c1-15(2,3)13(18)20-17(12-10-8-7-9-11-12)21-14(19)16(4,5)6/h7-11H,1-6H3
InChI key
DZKPLZUSZXYHFB-UHFFFAOYSA-N
Application
Reagent for:
- Preparation of α,β-unsaturated-γ-lactams via Rh-catalyzed C-H amination of allene carbamates, then Ru-catalyzed cyclocarbonylation
- Palladium-catalyzed diamination
- Preparation of functionalized bicyclic heterocyclic compounds by rhodium-catalyzed allene amidation and cyclization
- Preparation of piperidine and pyrrolidine derivatives via copper-catalyzed intramolecular aminoacetoxylation of aminoolefins
- C-H acyloxylation of arenes
- Hypervalent iodine oxidant
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
New! Rh2(esp)2, and exceptionally efficient and selective catalyst for C-H amination.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[Bis(trifluoroacetoxy)iodo]benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)
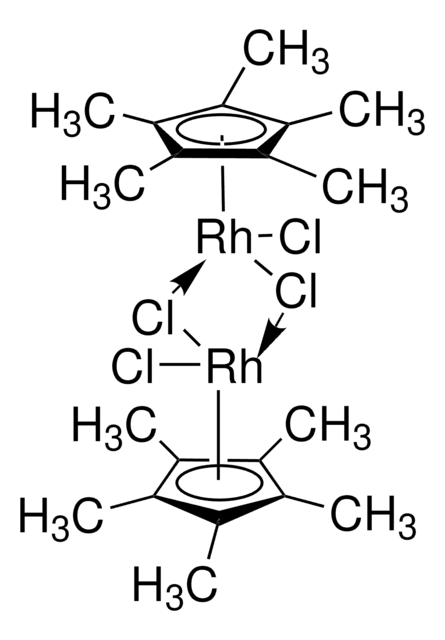
![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)

![[Hydroxy(tosyloxy)iodo]benzene 96%](/deepweb/assets/sigmaaldrich/product/structures/276/870/951f3ed1-f885-4305-aca0-303276ace392/640/951f3ed1-f885-4305-aca0-303276ace392.png)