719439
XtalFluor-E®
Synonym(s):
DAST difluorosulfinium salt, (Diethylamino)difluorosulfonium tetrafluoroborate, N,N-Diethyl-S,S-difluorosulfiliminium tetrafluoroborate, N,N-Diethylamino-S,S-difluorosulfinium tetrafluoroborate, N-(Difluoro-λ4-sulfanylidene)-N-ethyl-ethanaminium tetrafluoroborate
About This Item
Recommended Products
form
solid
Quality Level
mp
84-87 °C
functional group
amine
storage temp.
−20°C
SMILES string
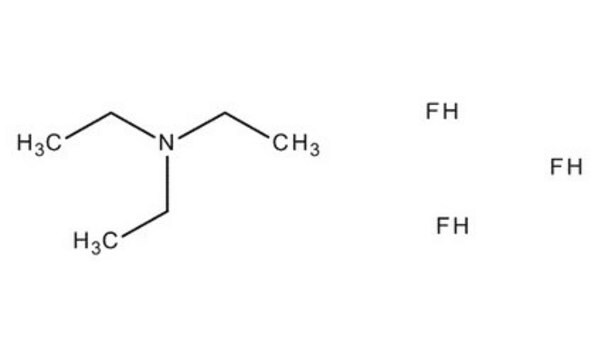
F[B-](F)(F)F.CC\[N+](CC)=S(\F)F
InChI
1S/C4H10F2NS.BF4/c1-3-7(4-2)8(5)6;2-1(3,4)5/h3-4H2,1-2H3;/q+1;-1
InChI key
YLNKFQWRRIXZPJ-UHFFFAOYSA-N
General description
Application
Deoxofluorination reagent with a better safety profile, that doesn′t generate corrosive HF which makes it suitable for use in standard borosilicate vessels, and does not react violently with water
Reactant for:
- Preparation of fluorodisaccharides
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
supp_hazards
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
This is an article regarding XtalFluor-E® and XtalFluor-M®: Convenient, Crystalline Deoxofluorination Reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service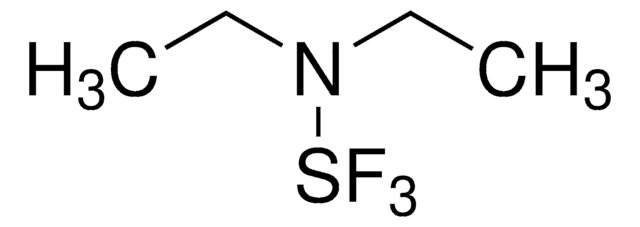
![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)