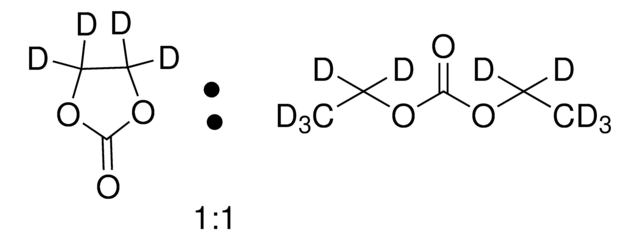
746746
Lithium hexafluorophosphate solution
in ethylene carbonate and diethyl carbonate, 1.0 M LiPF6 in EC/DEC=50/50 (v/v), battery grade
Synonym(s):
1.0 M LiPF6 EC/DEC=50/50 (v/v)
About This Item
Recommended Products
grade
battery grade
Quality Level
form
solution
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
(1.0 M LiPF6 in EC/DEC)
impurities
<15 ppm H2O
<50 ppm HF
color
APHA: <50
bp
130 °C
density
1.26 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤1 ppm
sulfate (SO42-): ≤2 ppm
cation traces
Ca: ≤1 ppm
Fe: ≤1 ppm
K: ≤1 ppm
Na: ≤1 ppm
Pb: ≤1 ppm
application(s)
battery manufacturing
greener alternative category
, Enabling
SMILES string
F[P-](F)(F)(F)(F)F.[Li+]
InChI
1S/F6P.Li/c1-7(2,3,4,5)6;/q-1;+1
InChI key
AXPLOJNSKRXQPA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
The ready-to-use electrolyte solutions are available in different solvent blends and can support a wide variety of lithium ion battery applications. These solutions are high purity and battery grade thus making them also suitable as standards in LIB research. Customized formulations can be made by inter-mixing the electrolyte solutions or by mixing appropriate of additives.
Other Notes
- Do not use with glass equipment
- All work should be done very quickly under dry air to prevent electrolytes from water uptake and solvent vaporization.
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT RE 1 Inhalation - STOT RE 2 Oral
target_organs
Bone,Teeth, Kidney
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
86.0 °F
flash_point_c
30 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Solid-state Li batteries: Review of solid electrolytes, ion conduction, structures, and electrochemical processes.
Li-ion batteries are currently the focus of numerous research efforts with applications designed to reduce carbon-based emissions and improve energy storage capabilities.
Lithium-ion batteries offer high energy density and cyclic performance for portable electronic devices.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Related Content
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service