902543
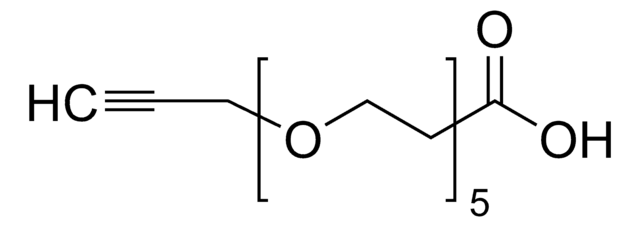
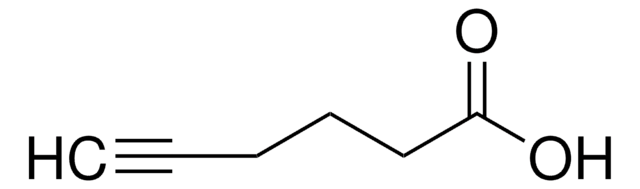
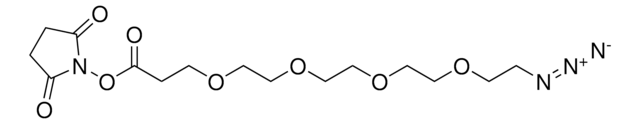
Propargyl-PEG4-acid
Synonym(s):
4,7,10,13-Tetraoxahexadec-15-ynoic acid, Propargyl-PEG4-CH2CO2H
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H20O6
CAS Number:
Molecular Weight:
260.28
MDL number:
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
form
liquid
reaction suitability
reagent type: linker
availability
available only in USA
refractive index
n/D 1.460
density
1.164 g/mL
functional group
alkyne
carboxylic acid
storage temp.
−20°C
SMILES string
OC(CCOCCOCCOCCOCC#C)=O
Application
This heterobifunctional, PEGylated crosslinker features a carboxylic acid at one end and propargyl group at the other for reaction with azide-containing compounds using click chemistry. The hydrophillic PEG linker facilitates solubility in biological applications. Propargyl-PEG4-acid can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Other Notes
Legal Information
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
related product
Product No.
Description
Pricing
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, Synthesis, and Evaluation of Novel 1,2,3-Triazole-Tethered Glycolipids as Vaccine Adjuvants.
Debabrata Bhunia et al.
Archiv der Pharmazie, 348(10), 689-703 (2015-09-04)
A Cu-mediated azide-alkyne click chemistry protocol was employed for the synthesis of a focused library of novel 1,2,3-triazolyl conjugates bearing various carbohydrate-steroid/triterpenoid entities. The immunogenicity of these compounds was examined initially by ex vivo assays. The lead compound 15g was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service