C86006
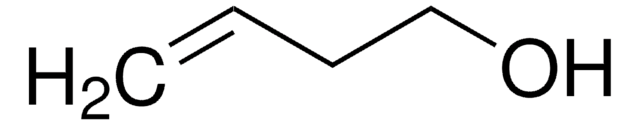
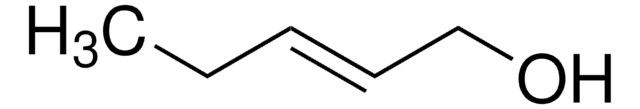
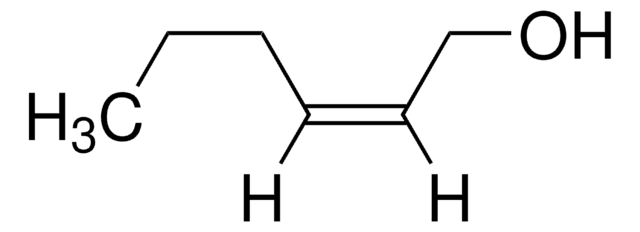
Crotyl alcohol, mixture of cis and trans
96%
Synonym(s):
2-Buten-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH=CHCH2OH
CAS Number:
Molecular Weight:
72.11
Beilstein/REAXYS Number:
1361395
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
liquid
refractive index
n20/D 1.427 (lit.)
bp
121-122 °C (lit.)
density
0.845 g/mL at 25 °C (lit.)
SMILES string
C\C=C\CO
InChI
1S/C4H8O/c1-2-3-4-5/h2-3,5H,4H2,1H3/b3-2+
InChI key
WCASXYBKJHWFMY-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Crotyl alcohol can be used as a starting material in the synthesis of antitumor agents such as 14-azacamptothecin and 10, 11-methylenedioxy-14-azacamptothecin. It can also be used as a precursor in the total synthesis of discodermolide.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
93.2 °F - closed cup
flash_point_c
34 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of optically active β-alkyl aspartate via [3, 3] sigmatropic rearrangement of α-acyloxytrialkylsilane.
Sakaguchi K, et al.
Tetrahedron Letters, 45(30), 5869-5872 (2004)
Total synthesis of discodermolide: optimization of the effective synthetic route.
de Lemos E, et al.
Chemistry?A European Journal , 14(35), 11092-11112 (2008)
Synthesis of 14-azacamptothecin, a water-soluble topoisomerase I poison.
Rahier N J, et al.
Organic Letters, 7(5), 835-837 (2005)
Evaluation of a role of acetaldehyde in the mechanism of inhibition of p-nitroanisole O-demethylation in isolated hepatocytes by ethanol.
E Dicker et al.
Archives of biochemistry and biophysics, 217(2), 441-451 (1982-09-01)
Darby R Schmidt et al.
Organic letters, 5(19), 3535-3537 (2003-09-12)
[structure: see text] A synthesis of the C(15)-C(30) fragment of Dolabelides A and B has been achieved. The recently developed asymmetric silane alcoholysis and tandem silylformylation-crotylsilylation reactions were used as the key steps to establish the C(23)-C(27) 1,5-syn-diol. In addition
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service