M66005
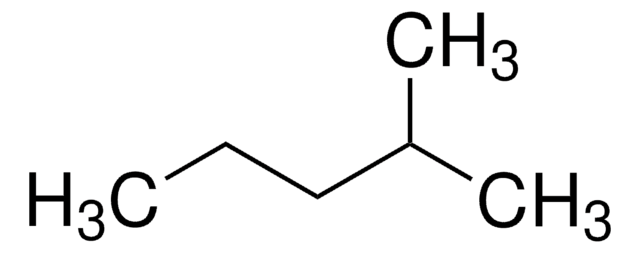
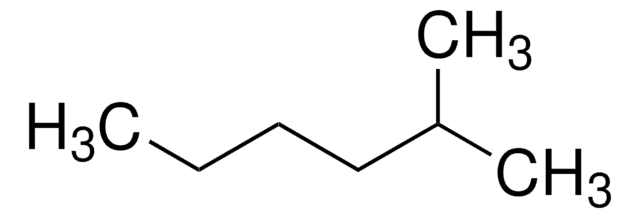
3-Methylpentane
≥99%
Synonym(s):
3-Methylpentane
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(2)
Select a Size
Change View
About This Item
Linear Formula:
(CH3CH2)2CHCH3
CAS Number:
Molecular Weight:
86.18
Beilstein/REAXYS Number:
1730734
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.97 (vs air)
Quality Level
vapor pressure
135 mmHg ( 17 °C)
assay
≥99%
form
liquid
autoignition temp.
532 °F
expl. lim.
~7.7 %
refractive index
n20/D 1.376 (lit.)
bp
64 °C (lit.)
density
0.664 g/mL at 25 °C (lit.)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Methylpentane (3MP) is mainly used as a model n-alkane in studies relating to densities, viscosities, refractive indices, excess enthalpies and vapor-liquid equilibrium of binary alkane mixtures.
signalword
Danger
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
19.4 °F - closed cup
flash_point_c
-7 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vapor-liquid equilibriums for the binary systems n-hexane with 2-methylpentane, 3-methylpentane, and 2, 4-dimethylpentane.
Ho C L and Davison R R
Journal of Chemical and Engineering Data, 24(4), 293-296 (1979)
Densities, viscosities, and refractive indices of the binary systems methyl tert-butyl ether+ 2-methylpentane,+ 3-methylpentane,+ 2, 3-dimethylpentane, and+ 2, 2, 4-trimethylpentane at 298.15 K.
Bouzas A, et al.
Journal of Chemical and Engineering Data, 45(2), 331-333 (2000)
Excess enthalpies for the binary systems n-octane with 2-methylpentane and 3-methylpentane.
Ameling W
Journal of Chemical and Engineering Data, 28(2), 184-186 (1983)
R Hutterer et al.
Biochimica et biophysica acta, 1323(2), 195-207 (1997-01-31)
Time-resolved fluorescence measurements were performed for a set of n-anthroyloxy fatty acids (n-AS; n = 2, 3, 6, 9, 12, 16) in both solvent and vesicle systems. The Stokes' shifts and the mean relaxation times calculated from the time-resolved emission
M W Grinstaff et al.
Science (New York, N.Y.), 264(5163), 1311-1313 (1994-05-27)
Halogenation of an iron porphyrin causes severe saddling of the macrocyclic structure and a large positive shift in the iron(III)/(II) redox couple. Although pre-halogenated iron(II) porphyrins such as Fe(TFPPBr8) [H2TFPPBr8, beta-octabromo-tetrakis(pentafluorophenyl)-porphyrin] are relatively resistant to autoxidation, they rapidly reduce alkyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service