T89605
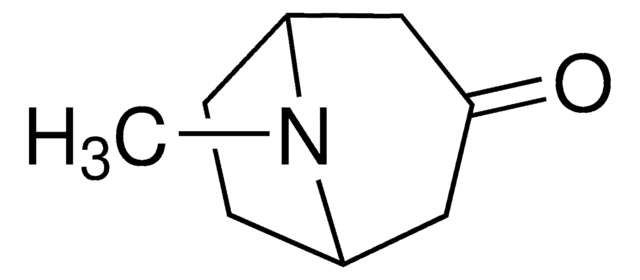
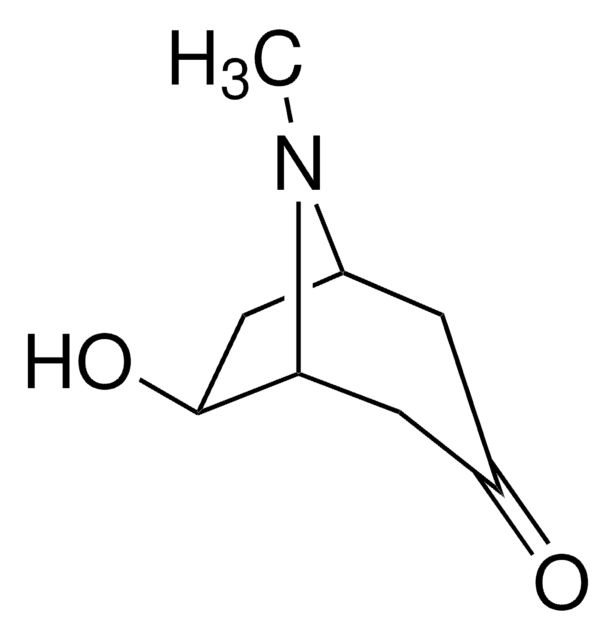
Tropinone
99%
Synonym(s):
8-Methyl-8-azabicyclo[3.2.1]octan-3-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H13NO
CAS Number:
Molecular Weight:
139.19
Beilstein/REAXYS Number:
2329
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
bp
113 °C/25 mmHg (lit.)
mp
40-44 °C (lit.)
storage temp.
2-8°C
SMILES string
CN1[C@@H]2CC[C@H]1CC(=O)C2
InChI
1S/C8H13NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-7H,2-5H2,1H3/t6-,7+
InChI key
QQXLDOJGLXJCSE-KNVOCYPGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
194.0 °F - closed cup
flash_point_c
90 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Portsteffen et al.
Phytochemistry, 37(2), 391-400 (1994-09-01)
In tropane-alkaloid producing plants and root cultures, the reduction of tropinone is a branch-point in secondary metabolism. Two different reductases stereospecifically form the isomeric alcohols tropine (tropan-3 alpha-ol) and pseudotropine (tropan-3 beta-ol). We describe here the purification and characterization of
Solid-phase synthesis of tertiary N-methyl amines including tropanes.
Michal Sienkiewicz et al.
Journal of combinatorial chemistry, 12(1), 5-8 (2009-11-03)
Jesús Marín-Sáez et al.
Journal of chromatography. A, 1518, 46-58 (2017-09-06)
Tropane alkaloids are a wide group of substances that comprises more than 200 compounds occurring especially in the Solanaceae family. The main aim of this study is the development of a method for the analysis of the principal tropane alkaloids
Andrea Brock et al.
The Plant journal : for cell and molecular biology, 54(3), 388-401 (2008-01-29)
Tropane alkaloids typically occur in the Solanaceae and are also found in Cochlearia officinalis, a member of the Brassicaceae. Tropinone reductases are key enzymes of tropane alkaloid metabolism. Two different tropinone reductases form one stereoisomeric product each, either tropine for
Tropane alkaloid biosynthesis. A century old problem unresolved.
A J Humphrey et al.
Natural product reports, 18(5), 494-502 (2001-11-09)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service